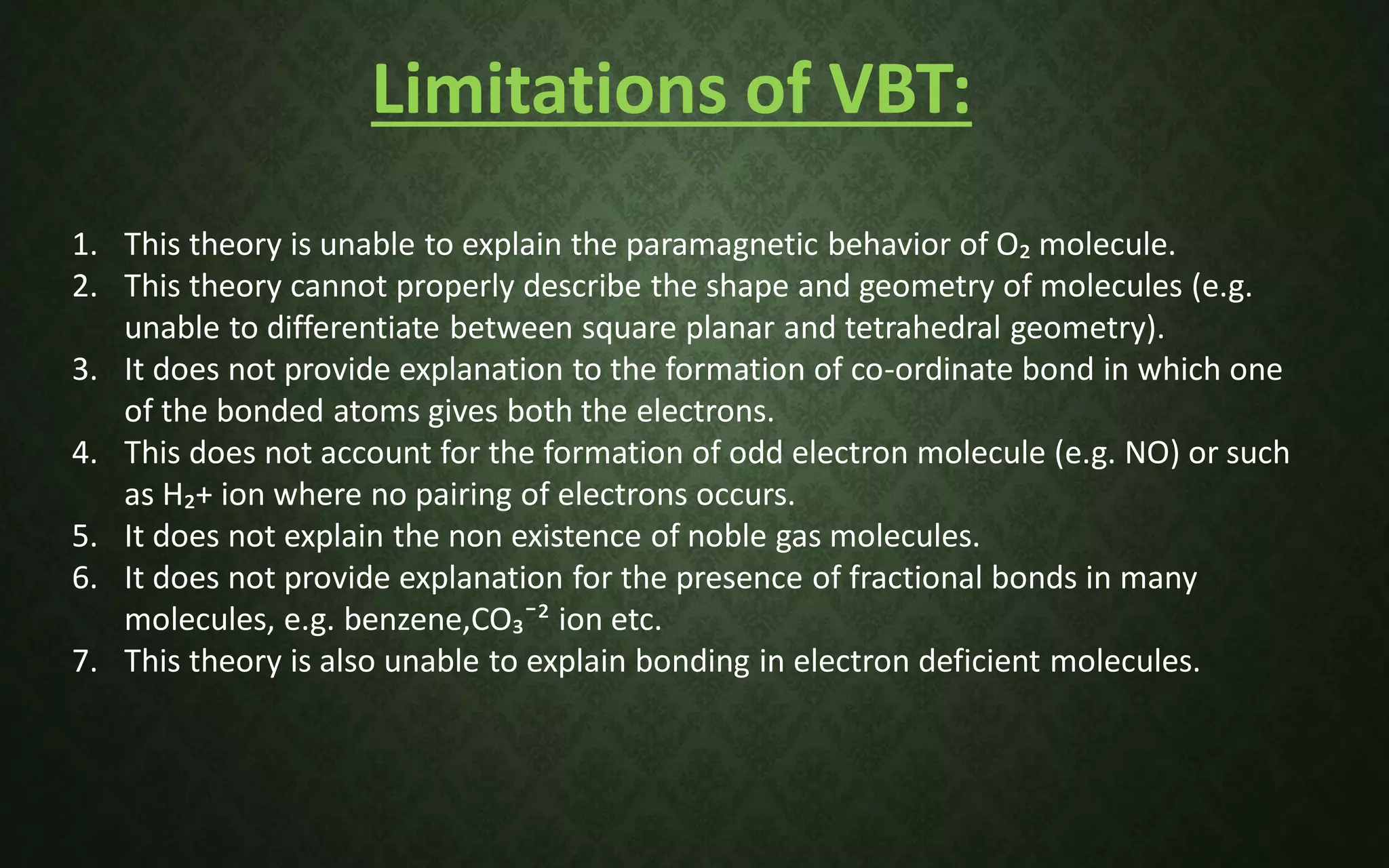
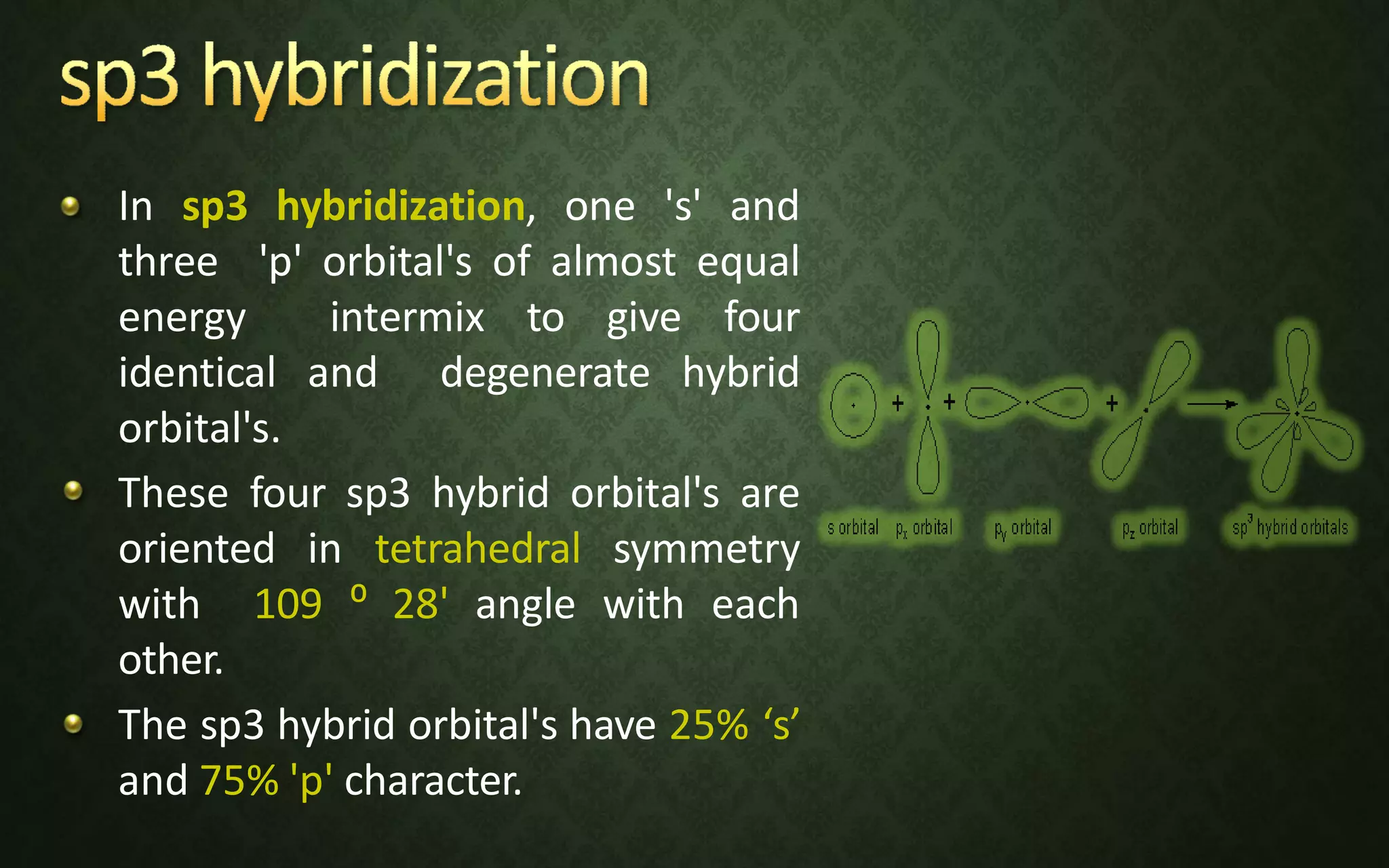
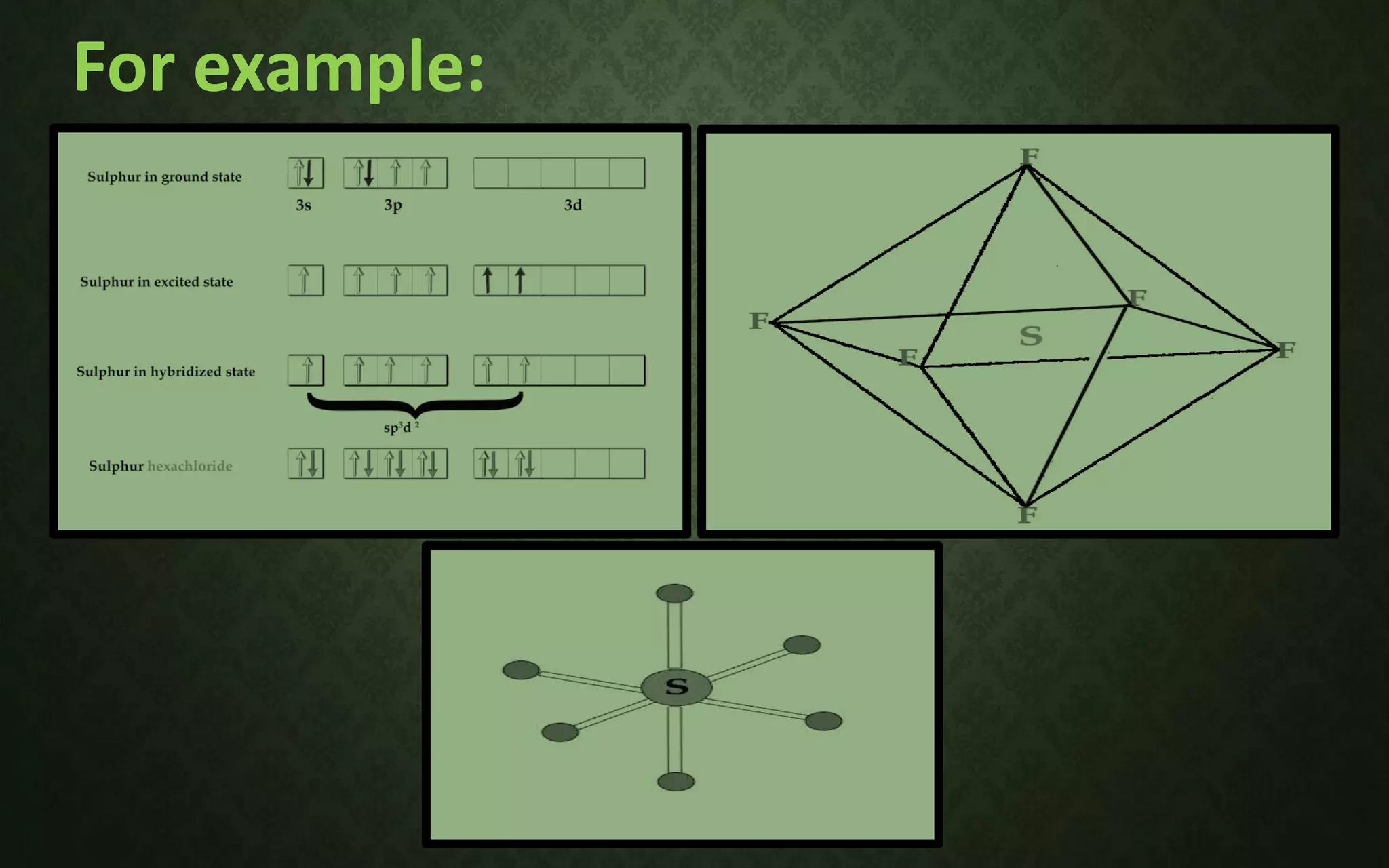
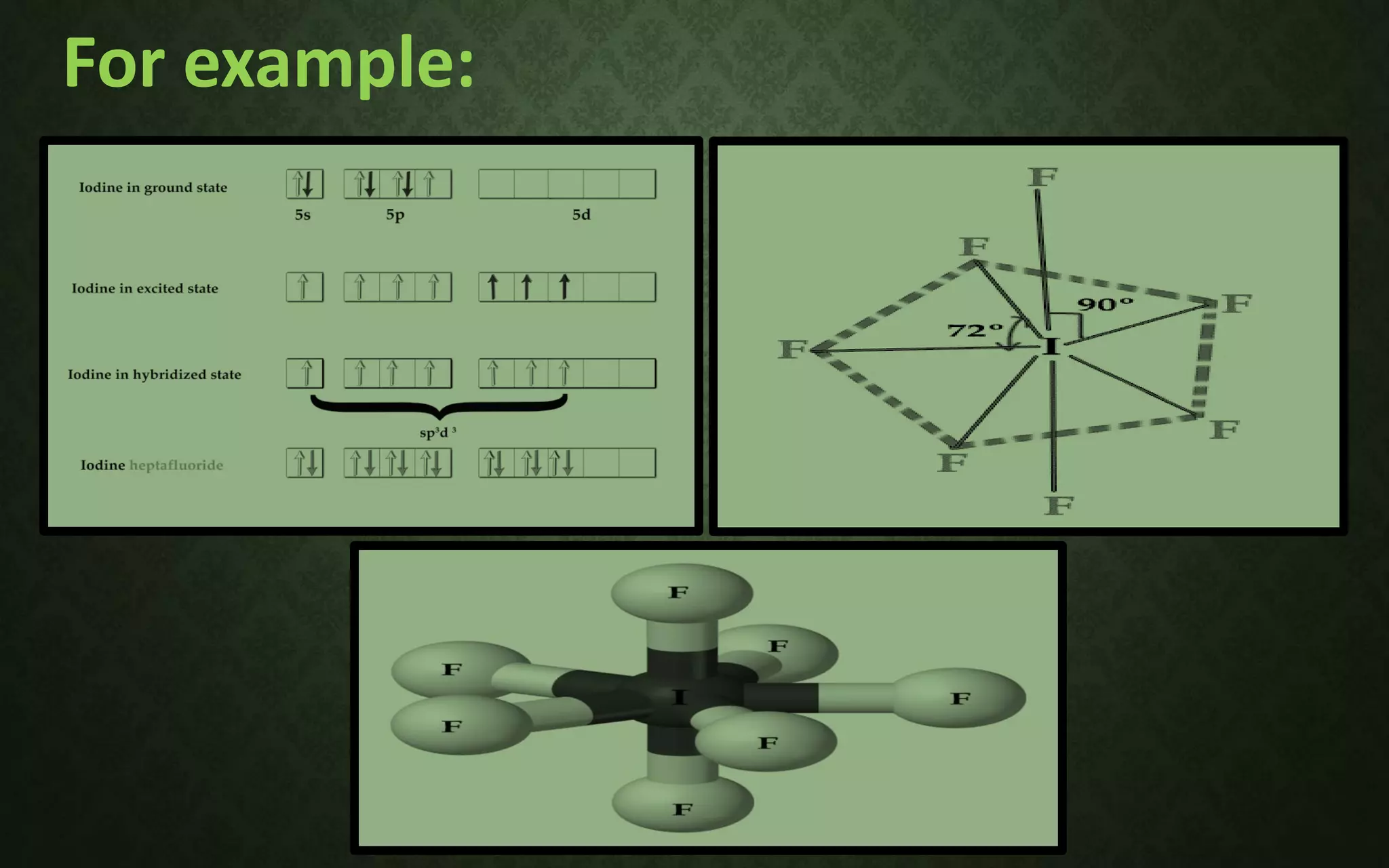
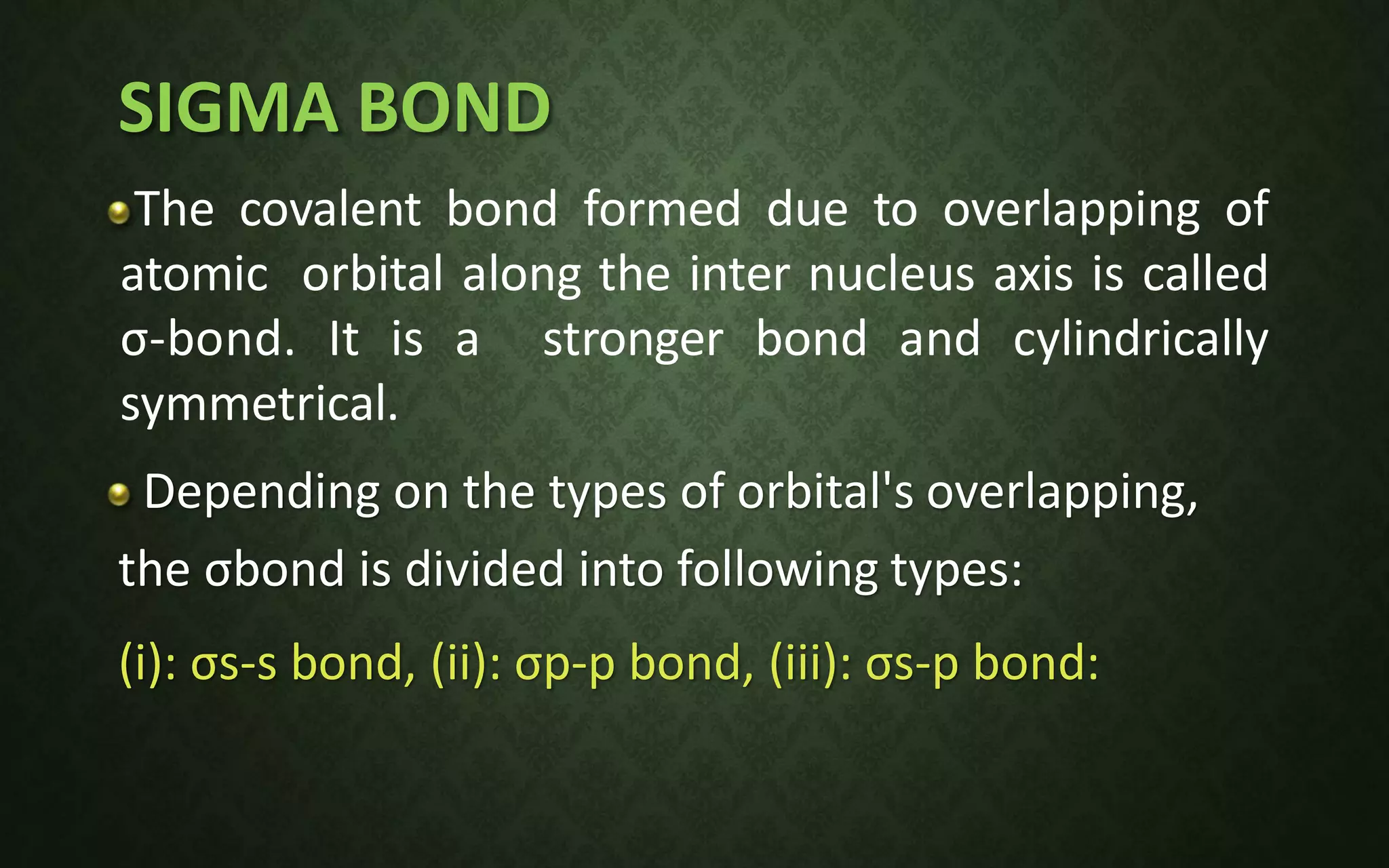
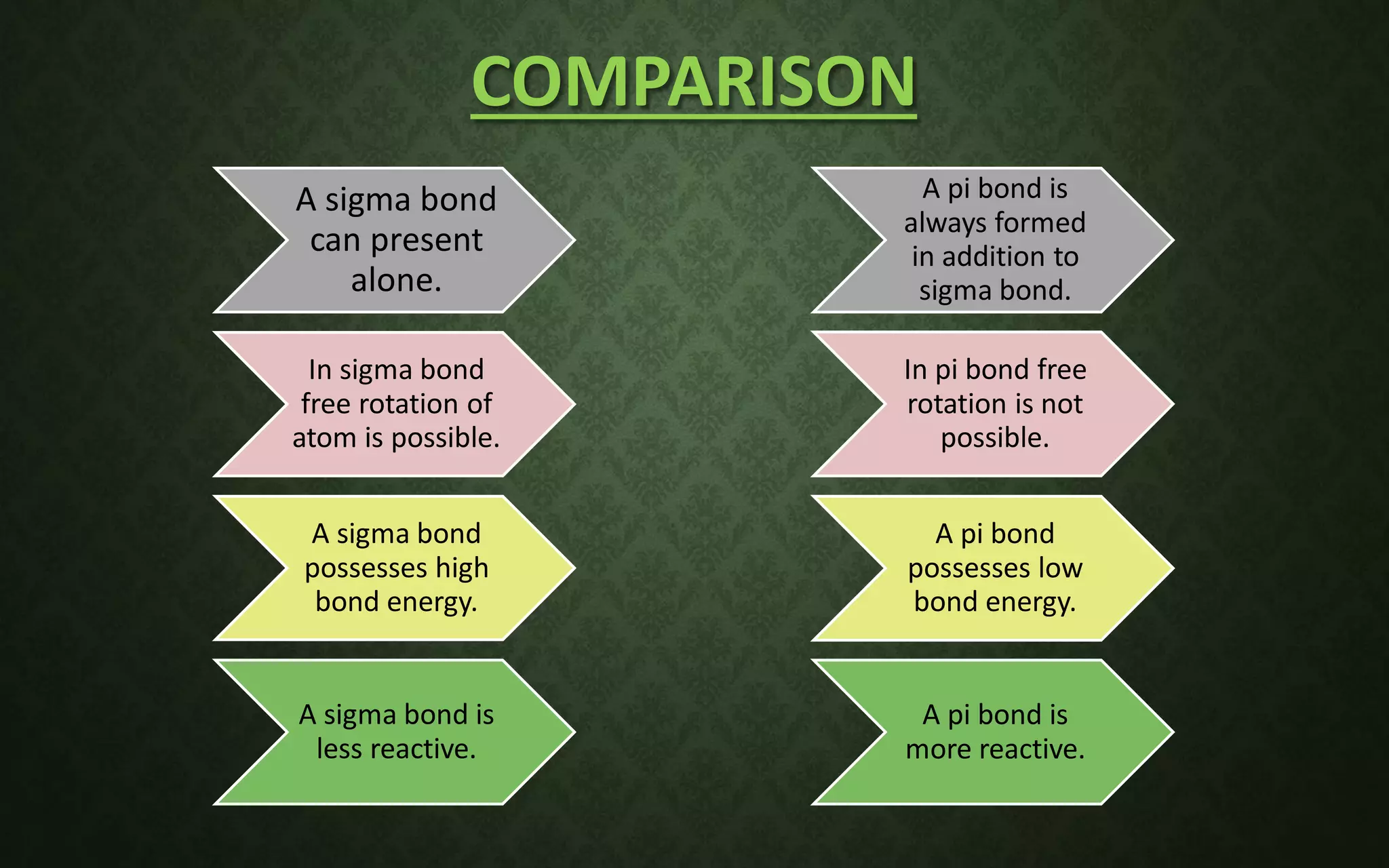
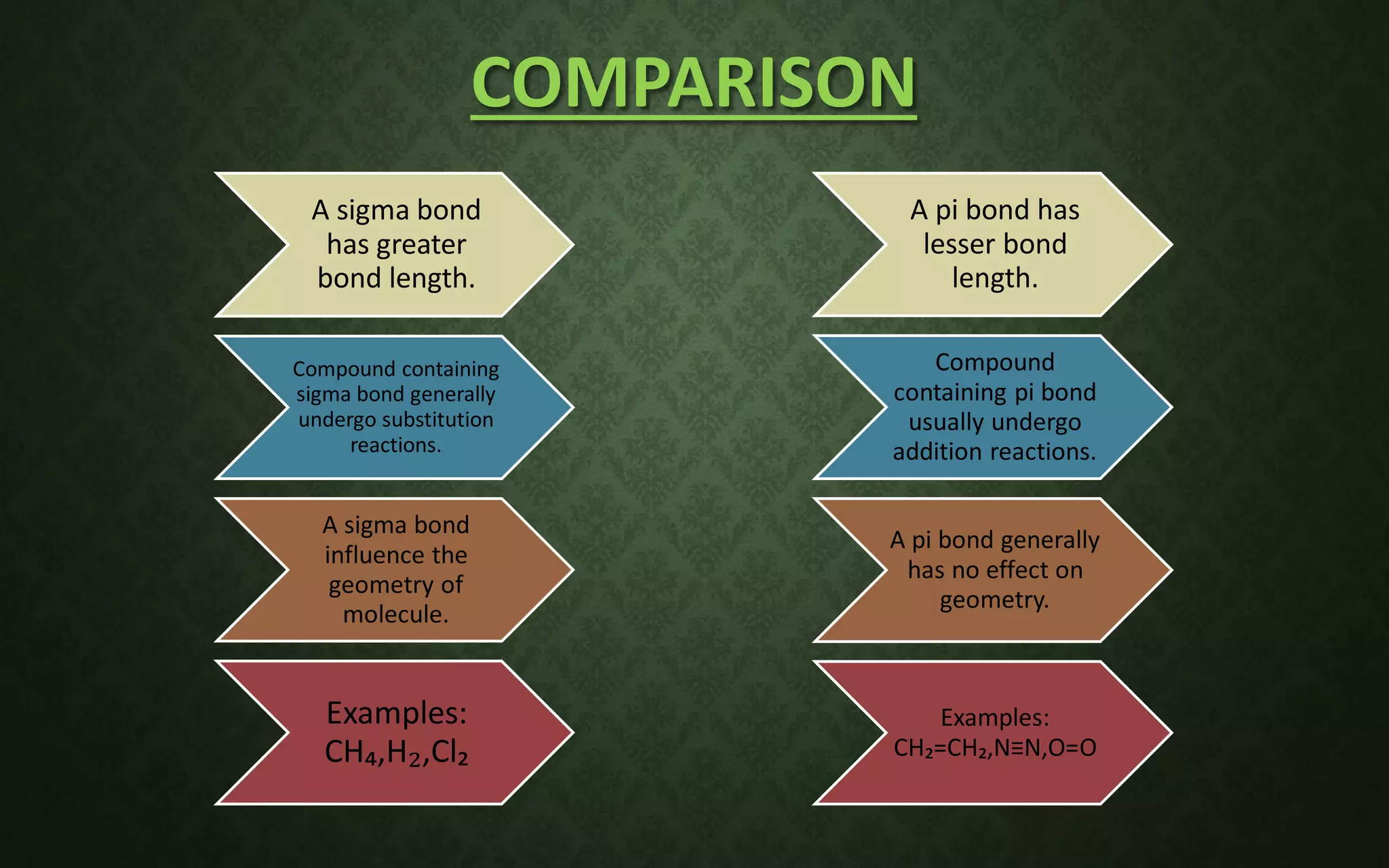
The document discusses valence bond theory and hybridization. It explains that valence bond theory describes how covalent bonds form through the overlapping of atomic orbitals to form sigma and pi bonds. It then defines different types of hybridization including sp, sp2, sp3, sp3d, sp3d2, and sp3d3 hybridization. These hybridization types involve the mixing of atomic orbitals to form new hybrid orbitals that determine molecular geometry. Examples are provided to illustrate different bond types and hybridization.














![The electronic configuration of Cl atom in the ground
state is [Ne]3s2 3px
2 3py
23pz
1.
The two half filled 3pz atomic orbital's of two chlorine
atoms overlap along the inter-nuclear axis and thus by
forming a σp-p bond.](https://image.slidesharecdn.com/vbtandhybridization-210124153413/75/Vbt-and-hybridization-18-2048.jpg)
![In the ground state, the electronic configuration of
hydrogen atom is 1s1 .
And the ground state electronic configuration of Cl
atom is [Ne]3s2 3px 2 3py 2 3pz 1 .
The half filled 1s orbital of hydrogen overlap with the
half filled 3pz atomic orbital of chlorine atom along the
internuclear axis to form a σs-p bond.](https://image.slidesharecdn.com/vbtandhybridization-210124153413/75/Vbt-and-hybridization-19-2048.jpg)
![The electronic configuration of O in the ground state is
[He] 2s2 2px 2 2py 1 2pz 1 .
The half filled 2py orbital's of two oxygen atoms
overlap along the inter-nuclear axis and form σp-p
bond.
The remaining half filled 2pz orbital's overlap laterally
to form a πp-p bond.](https://image.slidesharecdn.com/vbtandhybridization-210124153413/75/Vbt-and-hybridization-20-2048.jpg)

![The ground state electronic configuration of N is [He]
2s2 2px
1 2py
1 2pz
1.
Aσp-p bond is formed between two nitrogen atoms due
to overlapping of half filled 2px atomic orbital's along
the inter-nuclear axis.](https://image.slidesharecdn.com/vbtandhybridization-210124153413/75/Vbt-and-hybridization-22-2048.jpg)