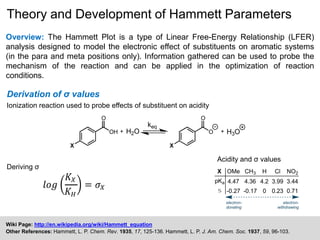
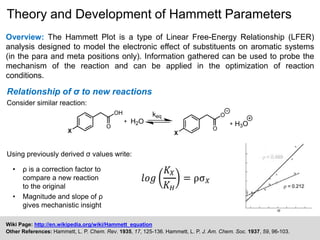
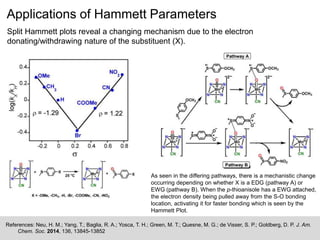
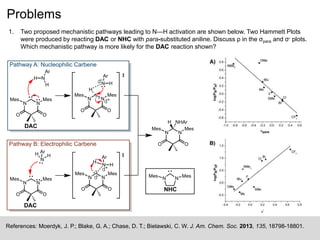
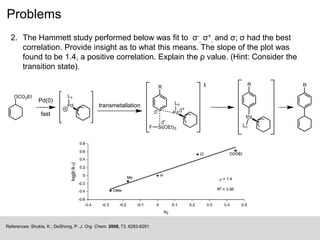
The document discusses the Hammett plot, which is a linear free-energy relationship analysis used to model the electronic effects of substituents on aromatic systems. It describes how σ values are derived from ionization reactions to indicate whether a substituent is electron-donating or electron-withdrawing. These σ values can then be used to analyze reaction mechanisms and optimize reaction conditions for similar processes. Examples are given of how split Hammett plots reveal changing mechanisms depending on the electronic nature of the substituent. Problems involving interpreting ρ values in Hammett plots to determine reaction pathways are also presented.