Organic Pedagogical Electronic Network
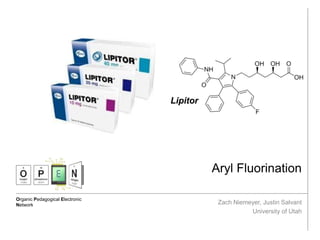
Aryl Fluorination
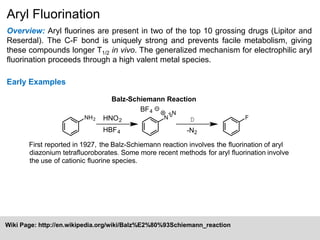
This document summarizes aryl fluorination, an important reaction for introducing fluorine groups onto aromatic rings. It notes that two of the top 10 grossing drugs, Lipitor and Reserdal, contain aryl fluorines. The mechanism proceeds through oxidation of an aryl group to a high valent metal species, followed by transmetalation and reductive elimination to introduce the fluorine. Examples are given of stoichiometric and catalytic aryl fluorination reactions using Selectfluor and silver oxide catalysts.
![Aryl Fluorination
Wiki Page: http://en.wikipedia.org/wiki/Balz%E2%80%93Schiemann_reaction
Overview: Aryl fluorines are present in two of the top 10 grossing drugs (Lipitor and
Reserdal). The C-F bond is uniquely strong and prevents facile metabolism, giving
these compounds longer T1/2 in vivo. The generalized mechanism for electrophilic aryl
fluorination proceeds through a high valent metal species.
Mechanism:
F
R
MX
R
Transmetalation
F+ source
Reductive
Elimination
[M]
M
R
Oxidation
M
R
F
High Valent Metal
N
N
F
Cl
2 PF6
-
N
F
N
N
F
Cl
2 BF4
-
SelectfluorF-TEDA-PF6
N-fluoro-2,4,6-
trimethylpyridinium
triflate](https://image.slidesharecdn.com/arylfluorination-170206164907/85/Aryl-fluorination-2-320.jpg)
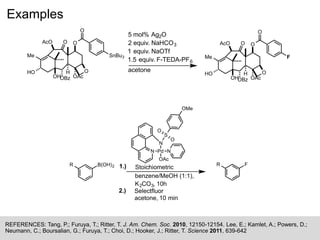
![Problems
References: Tang, P.; Furuya, T.; Ritter, T. J. Am. Chem. Soc. 2010, 12150-12154.
Assuming the catalytic cycle
shown, predict the product
formed upon the
addition of exogenous water:
F
R
MX
R
Transmetalation
F+ source
Reductive
Elimination
[M]
M
R
Oxidation
M
R
F
High Valent Metal
What is the role of NaOTf in the reaction below? (Hint: Ag2O is only partially soluble)
Me
O
O
O
O
SnBu3
HO
OBz
H
OAc
AcO
OH
Me
O
O
O
O
F
HO
OBz
H
OAc
AcO
OH
5 mol% Ag2O
2 equiv. NaHCO3
1 equiv. NaOTf
1.5 equiv. F-TEDA-PF6
acetone](https://image.slidesharecdn.com/arylfluorination-170206164907/85/Aryl-fluorination-5-320.jpg)
