Hammett Plots in the World of Enzymes
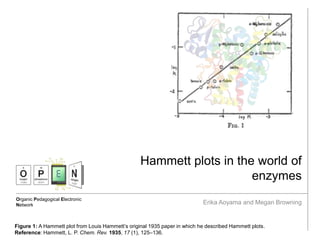
- 1. Organic Pedagogical Electronic Network Hammett plots in the world of enzymes Erika Aoyama and Megan Browning Figure 1: A Hammett plot from Louis Hammett’s original 1935 paper in which he described Hammett plots. Reference: Hammett, L. P. Chem. Rev. 1935, 17 (1), 125–136.
- 2. A substituent effect is the change in a molecule’s reactivity when a substituent on the molecule is changed. In 1935, Louis Hammett designed a scale to measure influence of various substituents (X) at the meta- or para- positions on the acidity of benzoic acid. He measured equilibrium constants of deprotonation of substituted benzoic acids and compared them to the equilibrium constant with X = hydrogen (unsubstituted benzoic acid). He called the log of this comparison sigma (σ). Either K (equilibrium constant) or k (rate constant) can be used in the equation: 𝑙𝑜𝑔 𝐾𝑥 𝐾 𝐻 = 𝑙𝑜𝑔 𝑘 𝑥 𝑘 𝐻 = 𝜎𝑥 Electron donating groups (EDG) have negative σ values (reduce acidity); electron withdrawing groups (EWG) have positive σ values (increase acidity). The relationship between reaction (or activation) free energy change and the character of the substituent is linear on a graph, so σ is called a linear free energy relationship (LFER). σ (sigma) References: Hammett, L. P. Chem. Rev. 1935, 17 (1), 125–136. Eric V. Anslyn; Dennis A. Dougherty. Modern Physical Organic Chemistry; University Science Books, 2006.
- 3. Hammett plots and ρ (rho) References: Hammett, L. P. Chem. Rev. 1935, 17 (1), 125–136. Eric V. Anslyn; Dennis A. Dougherty. Modern Physical Organic Chemistry; University Science Books, 2006. In general, if: ρ>0, negative charge is building (or positive charge is decreasing) • ρ>1: new reaction is more sensitive to substituents than benzoic acid reference reaction • 0<ρ<1: new reaction is less sensitive to substituents than the benzoic acid reference reaction ρ<0, positive charge is building (or negative charge is decreasing) in the reaction or the rate-limiting step (r.l.s.) of the reaction. For example, in this hydrolysis of an ester (see right), the 𝜌 value is 2.54. The positive sign indicates that the reaction creates negative charge like the deprotonation of benzoic acid, and the fact that 𝜌 is higher than 1 indicates it is more sensitive to substituents than benzoic acid. 𝜌 values have been used to elucidate mechanisms in all branches of chemistry. On the next few pages are two examples where Hammett plots were used to probe mechanisms for an enzyme-mimic- and enzyme catalyzed reactions. σ can be used to study substituent effects of other reactions by comparing 𝑙𝑜𝑔 𝑘 𝑥 𝑘 𝐻 of the new reaction to 𝜎𝑥 , to see if the new reaction is more or less sensitive to substituents than benzoic acid deprotonation. A graph of 𝑙𝑜𝑔 𝑘 𝑥 𝑘 𝐻 vs 𝜎𝑥 is called a Hammett plot. The slope of the Hammett plot is defined as 𝜌 (rho): 𝑙𝑜𝑔 𝐾 𝑥 𝐾 𝐻 = 𝑙𝑜𝑔 𝑘 𝑥 𝑘 𝐻 = 𝜌𝜎𝑥
- 4. In this paper, the researchers (Hoffmann et al.) used 𝜎+ to elucidate a mechanism for an enzyme mimic. They designed a catalyst to mimic tyrosinase, a copper containing enzyme that catalyzes the hydroxylation of phenols using dioxygen, a notoriously difficult reaction to catalyze synthetically. Hoffmann et al. made an synthetic catalyst enzyme-mimic that hydroxylates a variety of phenols at room temperature. They created a Hammett plot of their catalyst to compare it to the enzyme to see if their catalyst proceeded through a mechanism similar to tyrosinase. Using 𝜎+ for their Hammett plot, they calculated 𝜌 = -0.99, indicating that at the transition state, there is an increase in positive charge. This is consistent with the electrophilic aromatic substitution mechanism that is accepted for tyrosinase, and is also consistent with the trend of 𝜌 values published for tyrosinase: -1.8 to -2.2. The Hammett plot supported their claim that their catalyst acts through the same mechanism as tyrosinase. 𝜎+ and Biology example 1: Enzyme-mimic References: Eric V. Anslyn; Dennis A. Dougherty. Modern Physical Organic Chemistry; University Science Books, 2006. Okamoto, Y.; Brown, H. C. J. Org. Chem. 1957, 22 (5), 485–494. Hoffmann, A.; Citek, C.; Binder, S.; Goos, A.; Rübhausen, M.; Troeppner, O.; Ivanović- Burmazović, I.; Wasinger, E. C.; Stack, T. D. P.; Herres-Pawlis, S. Angewandte Chemie International Edition 2013, 52 (20), 5398–5401. Sigma only describes inductive charge stabilization/ destabilization effects (including indirect resonance effects). Other LFERs have been defined that describe other effects as well. In 1957, Herbert C. Brown sought an LFER that described effects of direct resonance charge stabilization. They used a new reference reaction in which the direct resonance stabilization of a positive charge far outweighs any inductive stabilization effects: This LFER is called 𝜎+. 𝜎+ is defined so that ρ<0 still means positive charge is building.
- 5. Biology example 2: Kynureninase-catalyzed reaction of β-benzoylalanine References: Kumar, S.; Gawandi, V. B.; Capito, N.; Phillips, R. S. Biochemistry 2010, 49 (36), 7913–7919. Kynureninase, an enzyme found in bacteria, catalyzes the following reaction, which is an important step in L-tryptophan catabolism: Kynureninase can catalyze a similar reaction involving β-benzoylalanine instead of L-kynurenine as the substrate: The rate-limiting part of the process is the release of the product L-Alanine. However, in this new reaction, the formation of the first product (benzoate) was found to be rate-limiting. To probe the mechanism of this new reaction, the authors synthesized derivatives of β-benzoylalanine with various substitutents (X) attached to the aromatic ring, used these derivatives as substrates for the kynureninase reaction, and created a Hammett plot from the rate data obtained (plot shown on next page).
- 6. References: Kumar, S.; Gawandi, V. B.; Capito, N.; Phillips, R. S. Biochemistry 2010, 49 (36), 7913–7919. Hammett plot for kynureninase-catalyzed reaction of substituted β-benzoylalanines. The break in the plot shows 2 different rate-limiting steps (r.l.s.) in benzoate formation, depending on X. Positive slope ρ on the left side of the plot shows that negative charge is building (or positive charge is decreasing) in the r.l.s. when X = EDG. Negative slope ρ on the right side of the plot shows that positive charge is building (or negative charge is decreasing) in the r.l.s. when X = EWG. Part of the proposed mechanism for benzoate formation. Step A has negative charge building, whereas Step B has a decrease in negative charge. This is consistent with the Hammett plot. The authors conclude that their Hammett plot provides support for a gem-diolate intermediate in the formation of benzoate, with Step A being the r.l.s. for X = EDG, and Step B being the r.l.s. for X = EWG. Step A Step B
- 7. Problems 1. Would X= MeO have a a. zero σ value b. positive σ value c. negative σ value 2. Why was σ insufficient and σ+ invented? a. σ described only resonance, and parameter including induction was needed b. σ described only induction and indirect resonance, and a parameter including direct resonance was needed c. σ described resonance and induction, and a parameter describing only induction was needed d. σ described direct resonance, and a parameter describing indirect resonance was needed 3. Is 𝜌 value positive or negative when the reactant containing the aromatic ring is acting as an electrophile? a. negative b. positive c. neither For 4 and 5 use the reaction to the right: 4. Which LFER parameter would best represent the reaction? a. σ- b. σ c. σ+ d. None of the above 5. For the reaction is 𝜌 a. Negative b. Positive c. Zero
- 8. This work is licensed under a Creative Commons Attribution- ShareAlike 4.0 International License. Contributed by: Erika Aoyama and Megan Browning University of Utah, 2016