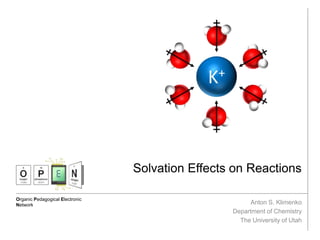
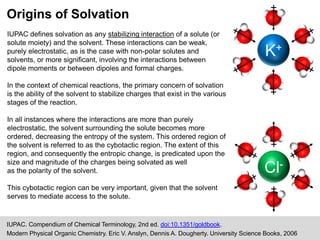
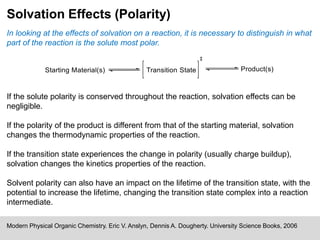
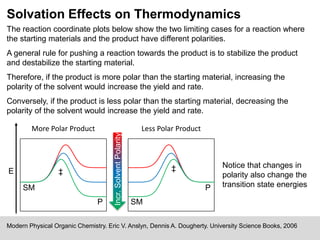
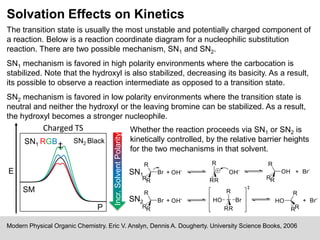
The document discusses solvation, defined as the stabilizing interactions between solute and solvent, which can significantly impact chemical reactions by influencing their thermodynamics and kinetics. It outlines various types of intermolecular interactions like van der Waals, dipole-dipole, and hydrogen bonding, and describes how solvent polarity affects reaction rates and the stability of transition states. Furthermore, it highlights the importance of the cybotactic region in mediating solute access and exemplifies its role in peptide folding in mixed solvent environments.