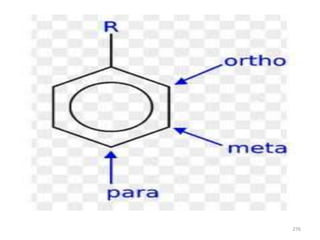
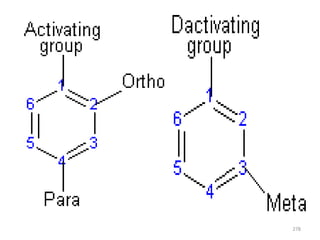
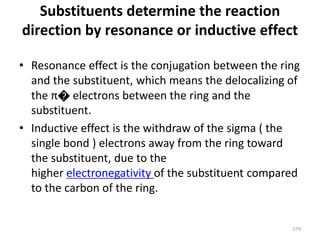
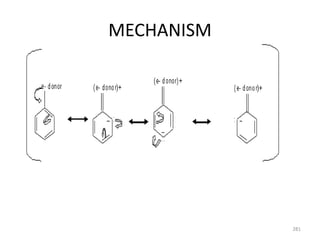
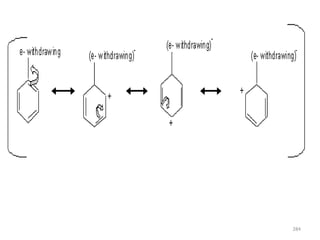
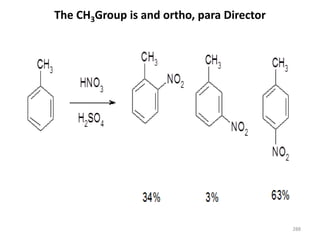
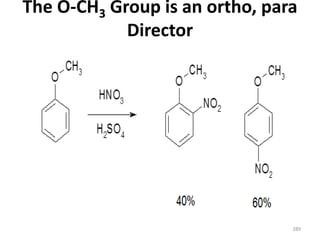
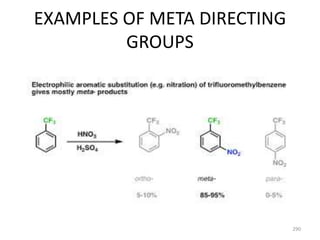
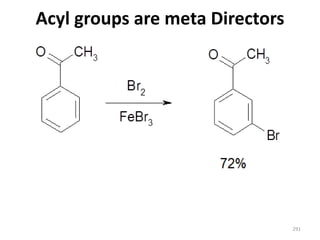
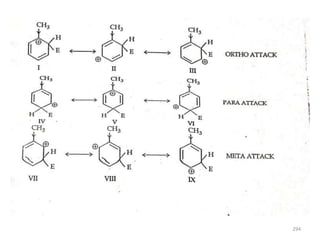
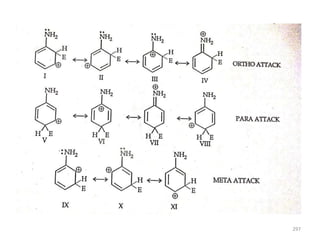
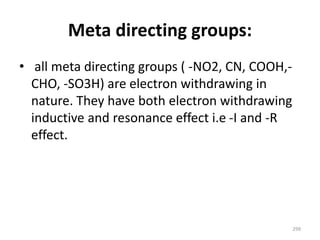
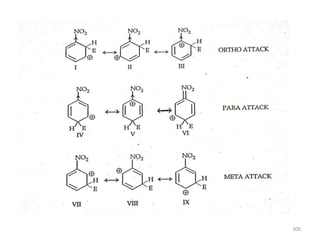
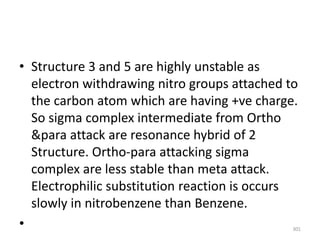
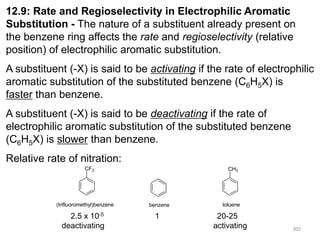
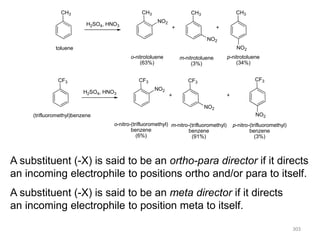
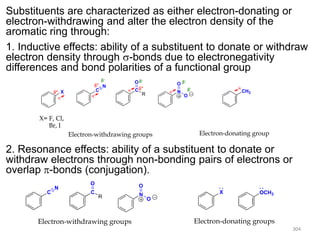
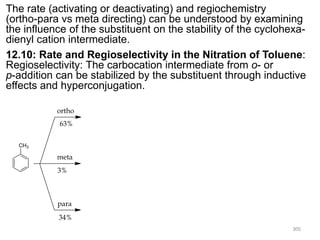
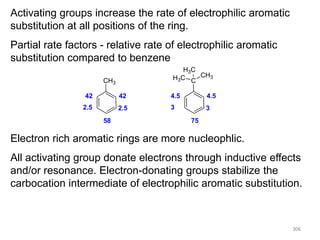
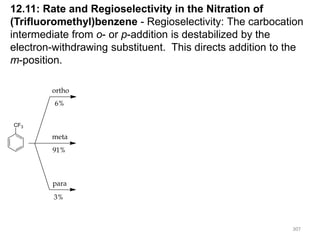
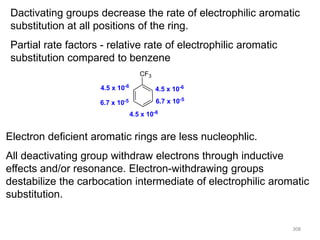
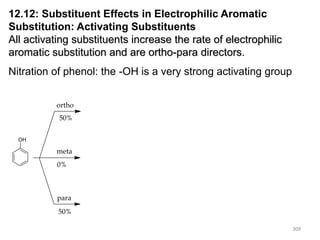
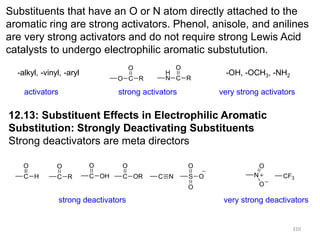
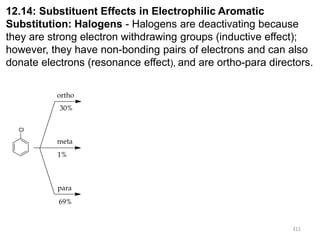
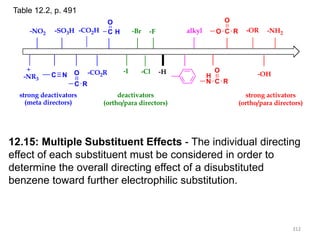
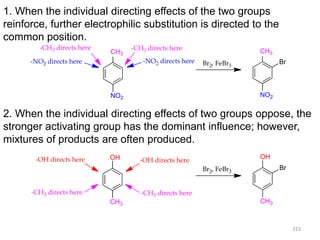
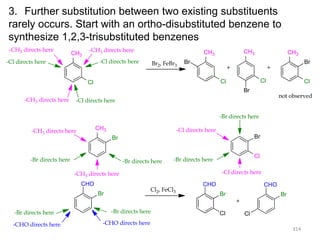
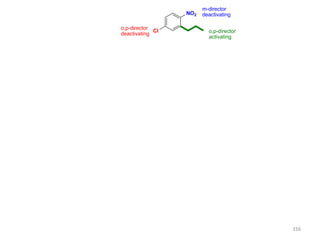
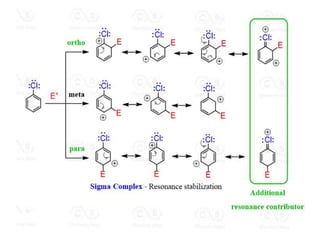
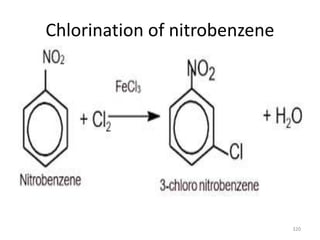
Electrophilic aromatic substitution reactions on benzene rings are influenced by the orientation and directing effects of existing substituents. Activating groups that donate electron density through inductive or resonance effects direct incoming electrophiles to the ortho and para positions. Deactivating groups withdraw electron density through inductive effects and direct electrophiles to the meta position, with the exception of halogens which are also ortho, para directors due to their ability to stabilize carbocation intermediates through resonance. The rate and products of electrophilic aromatic substitutions can be understood and predicted based on the individual directing effects of substituents, their ability to stabilize or destabilize carbocation intermediates, and their combined influences on the regioselect
















![321
12.17: Substitution in Naphthalene (please read)
12.18: Substitution in Heterocyclic Aromatic Compounds
(please read)
Summary of electrophilic aromatic substitution of benzene
Zanger, M.; Gennaro, A. R.; McKee, J. R. J. Chem. Ed. 1993, 70 (12) , 985-987
SO3H X
(m-) (o-, p-)
NO2 CH2R
O R
(o-, p-)
(m-) (m-)
Br R
(o-, p-)
CO2H
(m-)
HNO3,
H2SO4
X2,
catalyst
RCH2X,
AlCl3
RCOCl,
AlCl3
SO3,
H2SO4
[O]
NBS,
h [O]
[H]](https://image.slidesharecdn.com/orientationinaromaticcompounds-230331044629-cd8cb2e7/85/Orientation-in-Aromatic-compounds-ppt-47-320.jpg)