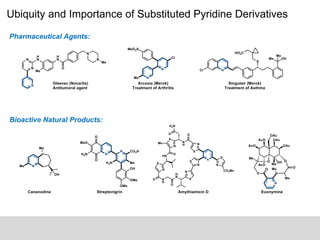
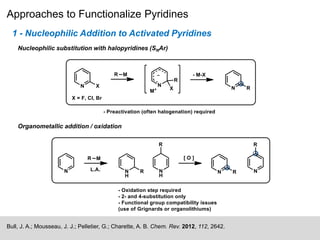
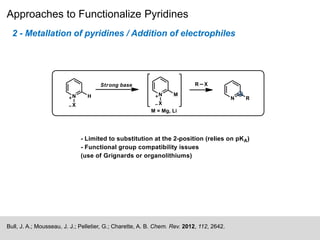
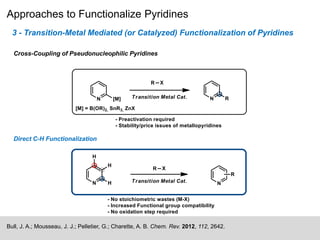
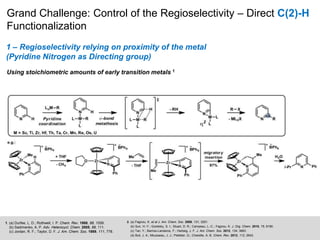
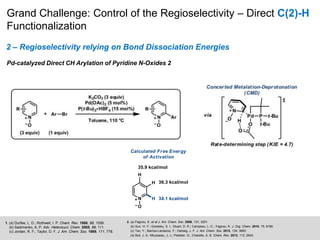
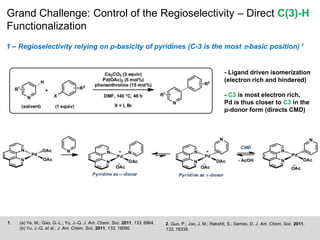
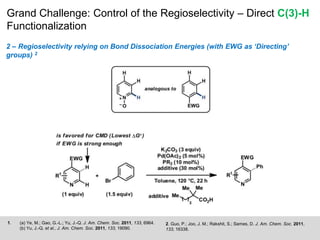
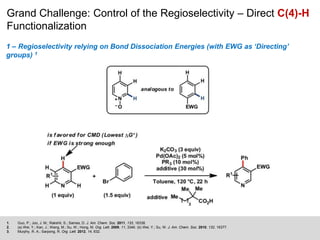
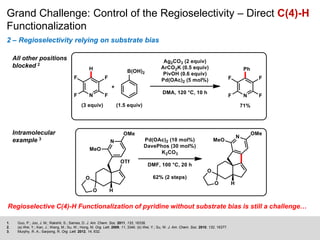
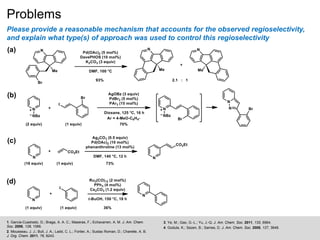
This document discusses the functionalization strategies for pyridine derivatives vital in pharmaceutical applications, emphasizing the significance of regioselectivity during direct C-H functionalization. Various approaches, including nucleophilic addition, metallation, and transition-metal mediated reactions, are detailed, along with the challenges involved in achieving desired regioselectivity. The work is associated with the Sarpong Lab at UC Berkeley and is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.