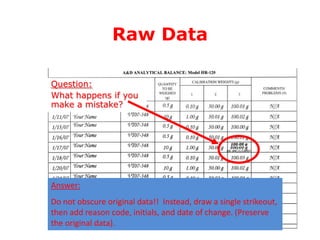
Good Manufacturing Practices (GMP) ensure consistency in the quality of pharmaceutical products. GMP guidelines cover quality control, documentation, personnel qualifications, premises, equipment, materials, production, and quality assurance. Adherence to GMP is important for pharmaceutical export and most regulatory agencies only approve imports of medicines made under GMP standards. Key aspects of GMP include qualification and validation of equipment and processes, complaint handling, recalls, and self-inspections. GMP guidelines also exist specifically for herbal medicines to ensure quality, safety and efficacy of these complex biological products.