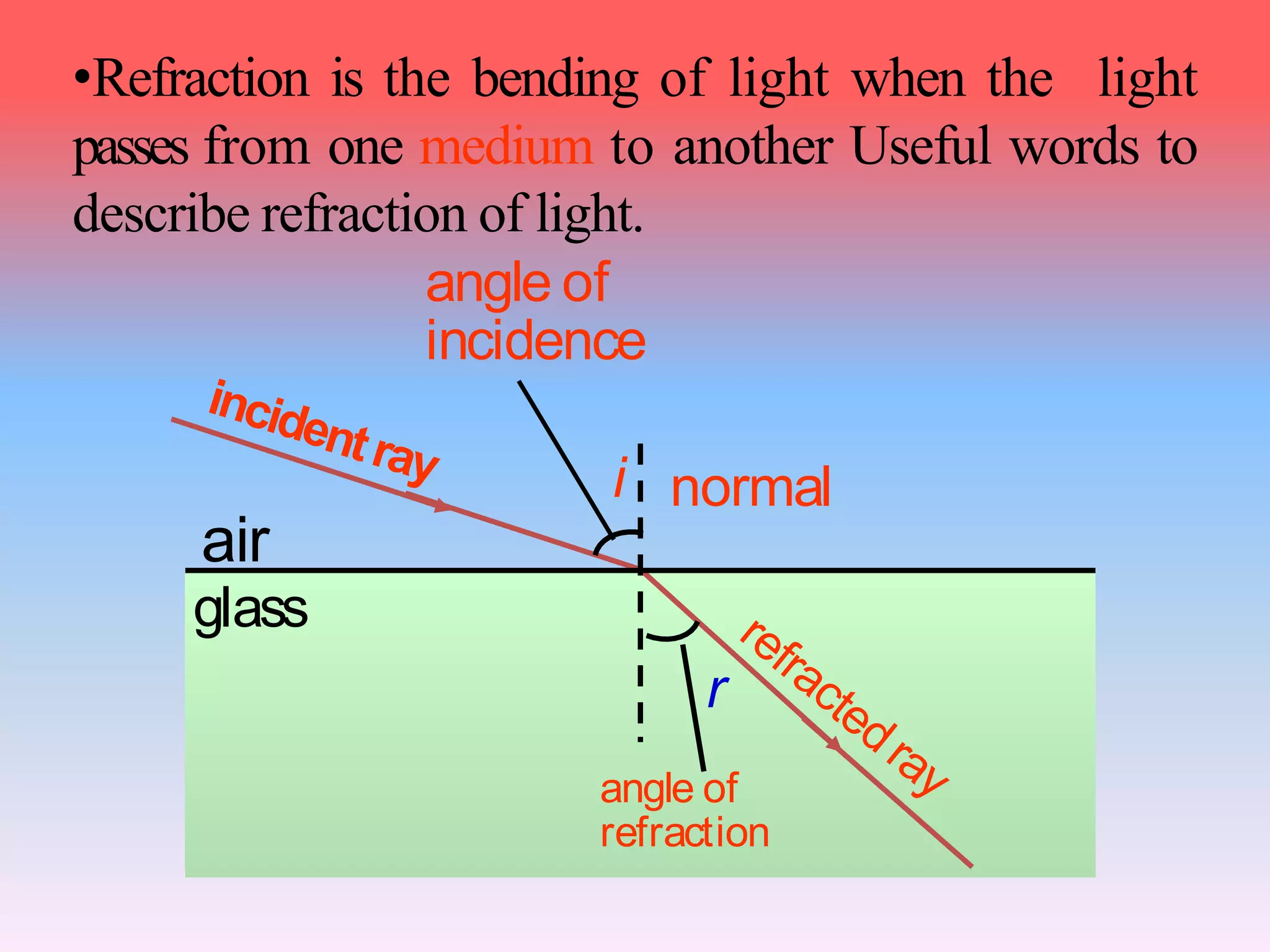
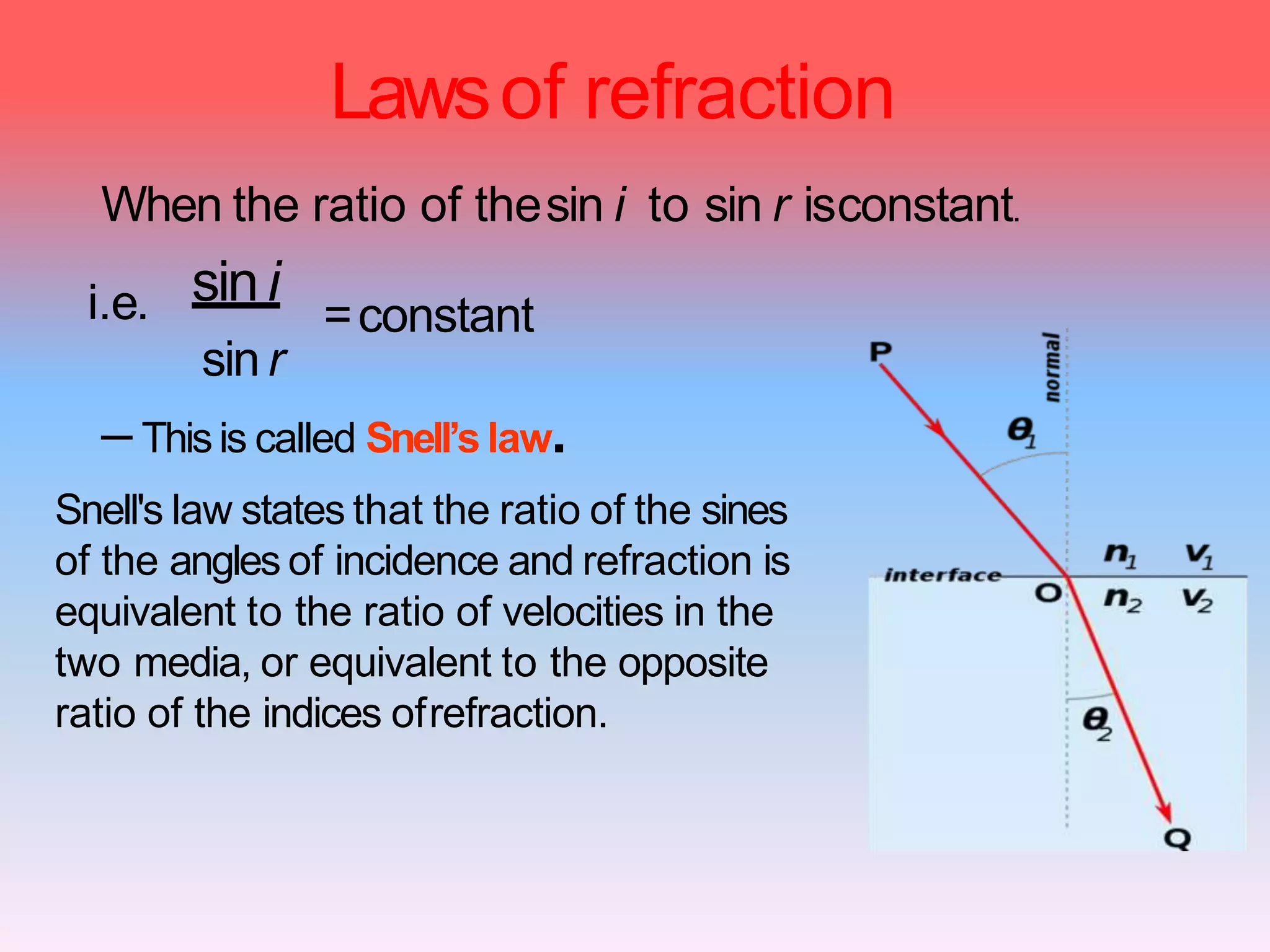
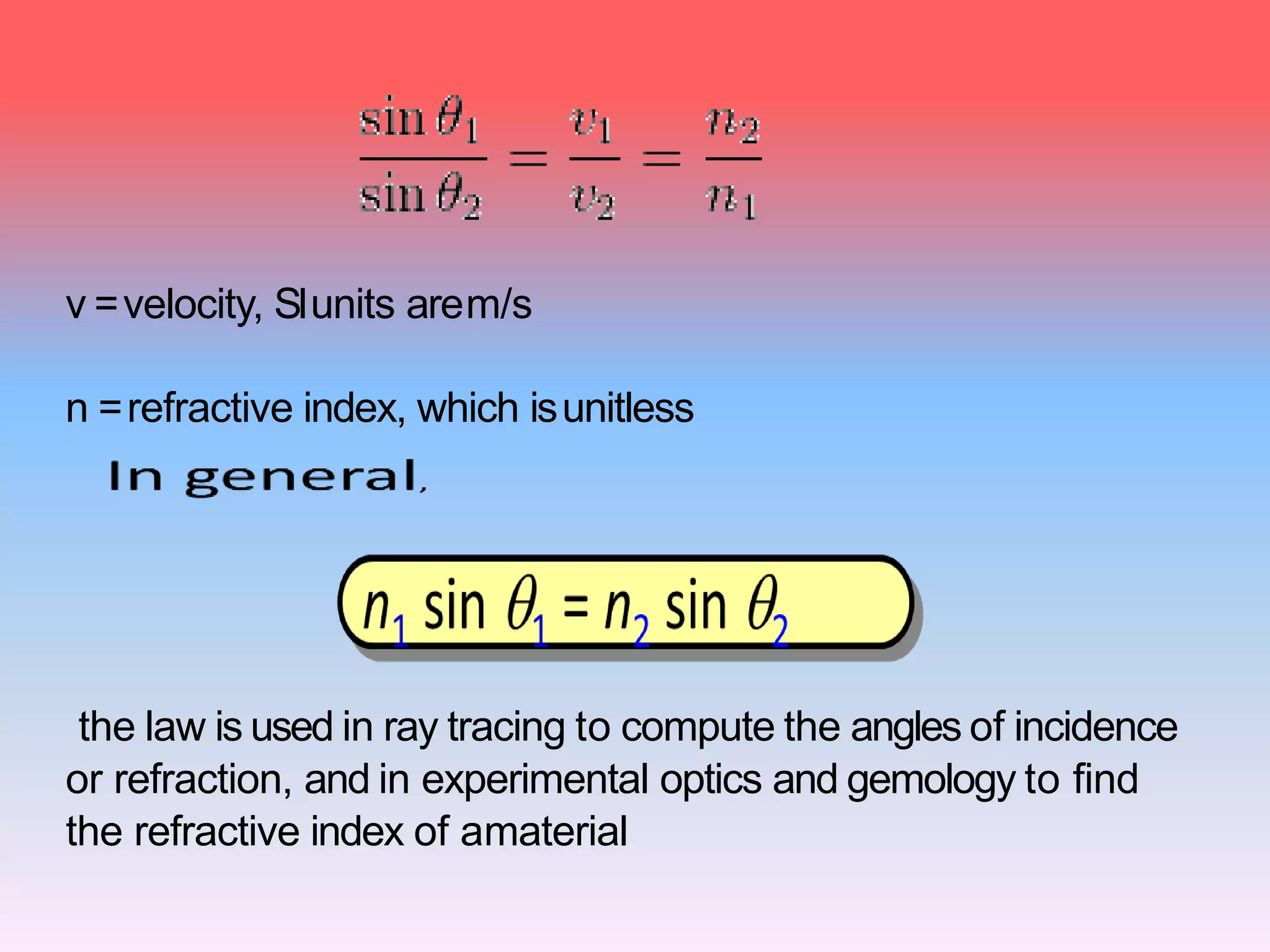
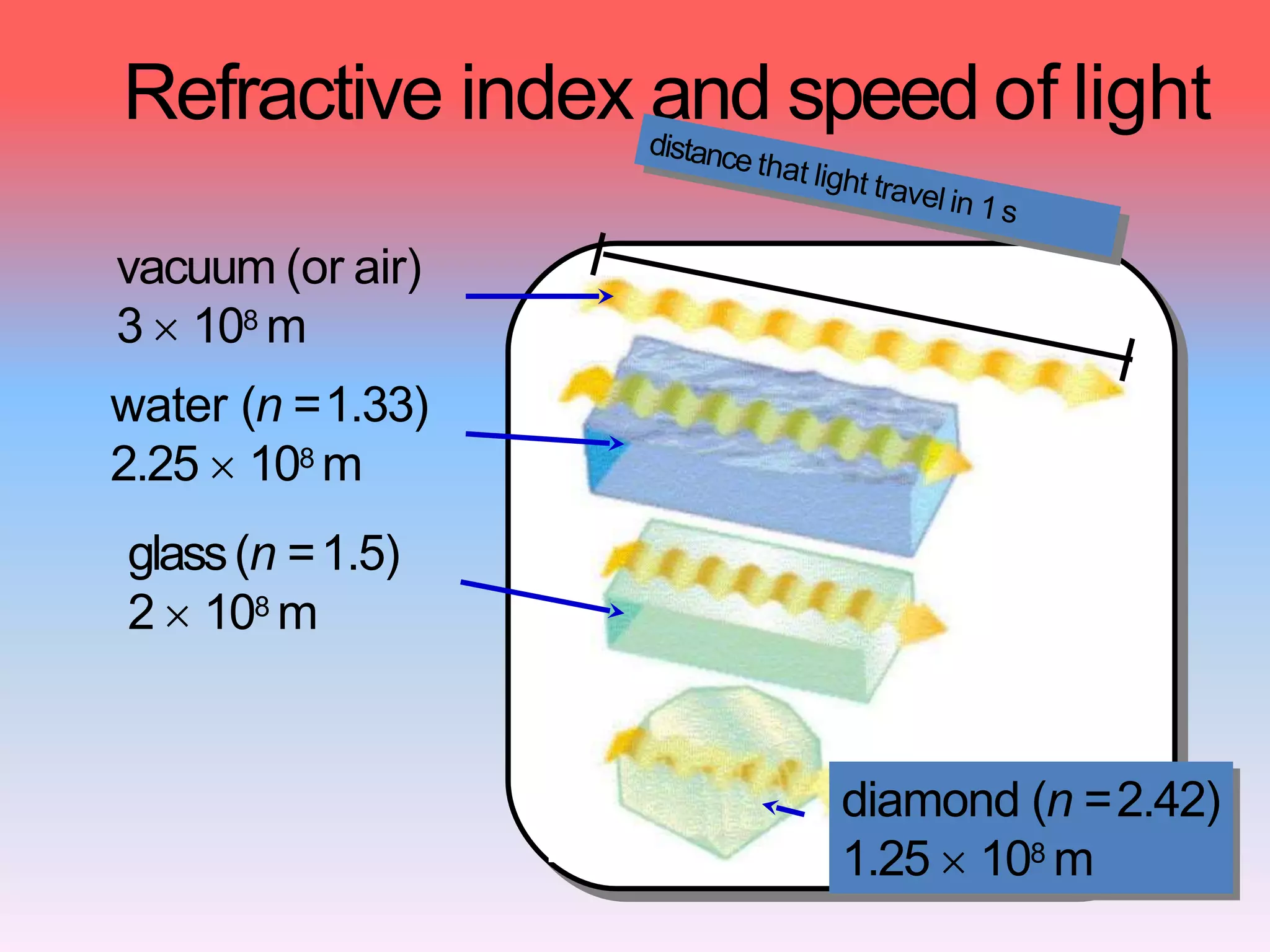
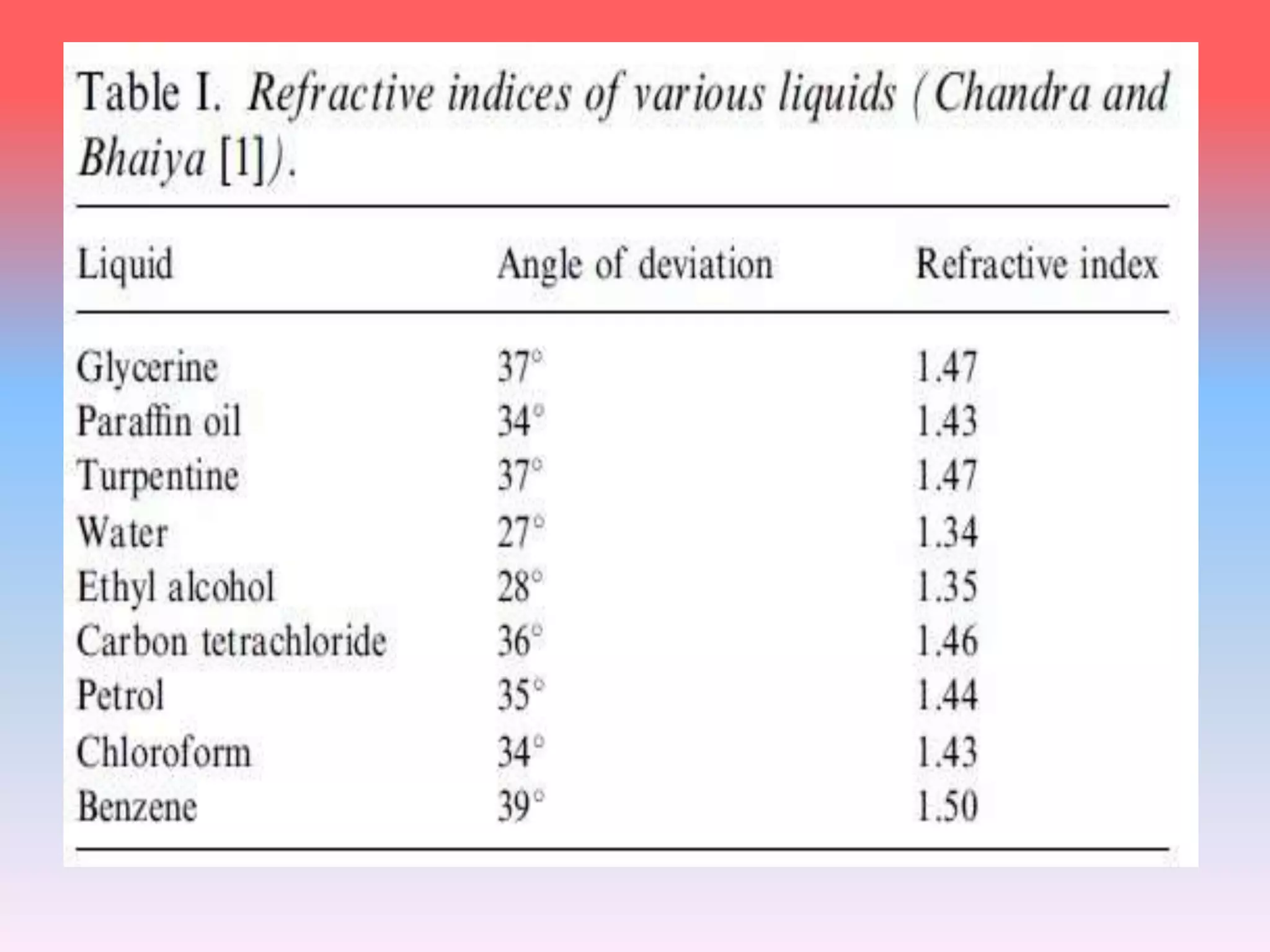
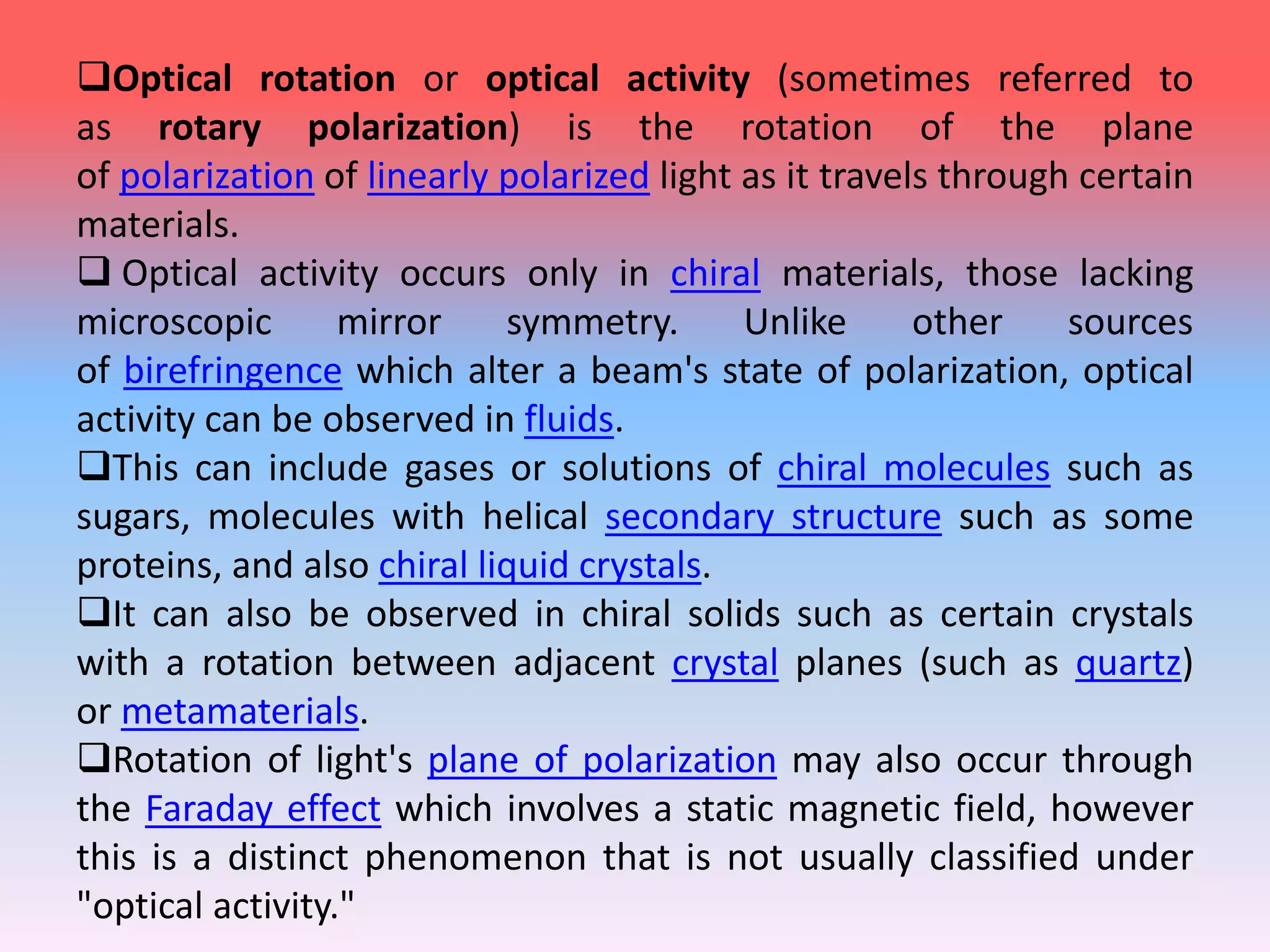
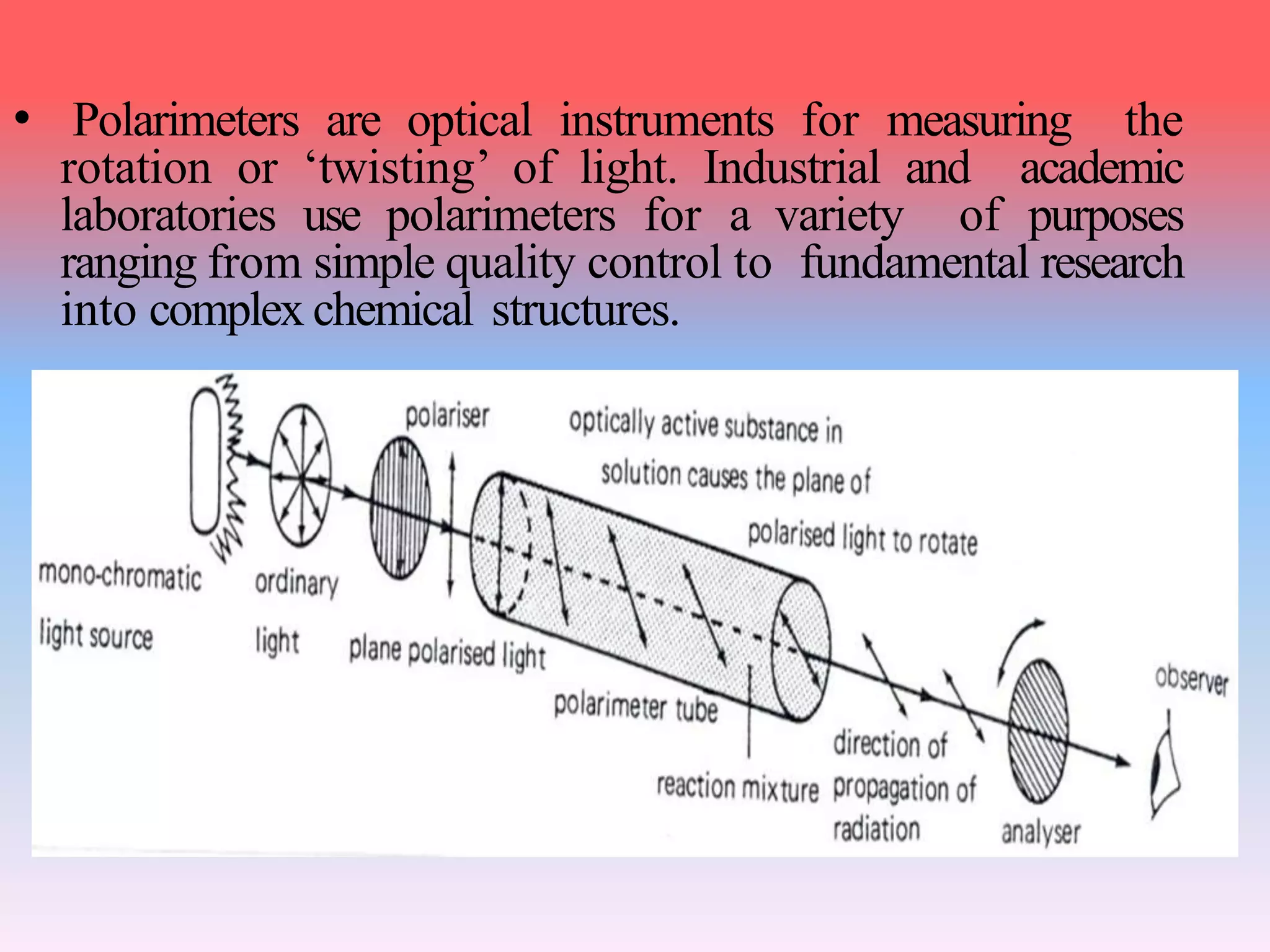
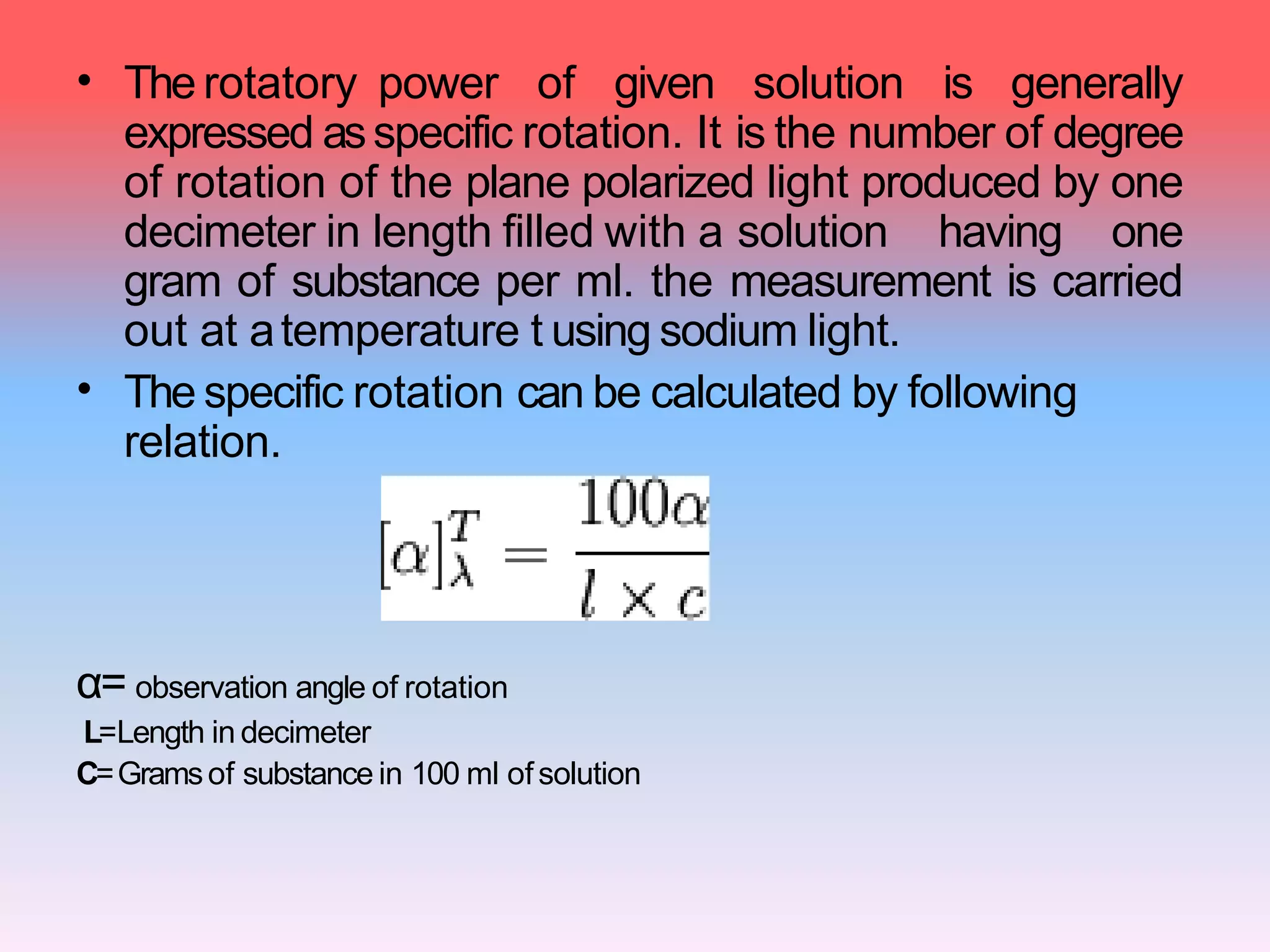
This document discusses refractive index, optical rotation, and dissociation constant. It defines refractive index as a ratio of the speed of light in a vacuum to the speed of light in a medium. It explains that optical rotation is the rotation of the plane of polarized light when it passes through certain chiral materials. It also defines dissociation constant as a quantity expressing the extent to which a substance dissociates into ions in solution. Various techniques for measuring refractive index and applications of optical rotation and dissociation constants are also summarized.