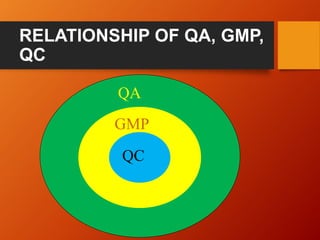
This document provides an overview of key elements and basic principles of Good Manufacturing Practices (GMP). It defines GMP and cGMP, outlines their importance in ensuring product quality and safety, and describes characteristics of GMP-compliant products. The document also summarizes key elements of GMP like personnel, premises, equipment, materials, and quality control. It emphasizes the importance of documentation, validation, recalls, self-inspection, storage and training in adhering to GMP. The objectives of GMP and guidelines from various regulatory bodies are also briefly mentioned.