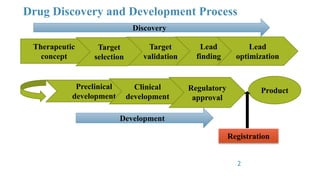
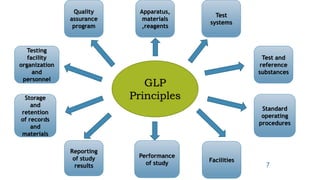
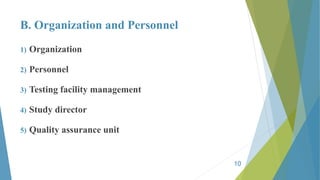
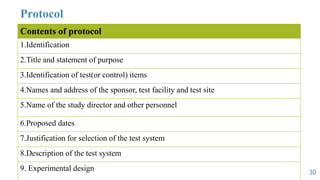
GLP principles were created by the USFDA and OECD to establish standardized practices in non-clinical laboratory studies due to past malpractices. GLP aims to ensure study data accurately reflects results and is traceable by regulating an organization's quality system, facilities, equipment, test systems, operating procedures, personnel qualifications, protocols, records, reports and archiving. Key aspects of GLP include establishing responsibilities for management, study directors and quality assurance units and having standard operating procedures for conducting studies, testing facilities, equipment, reagents and animal care in accordance with approved protocols.