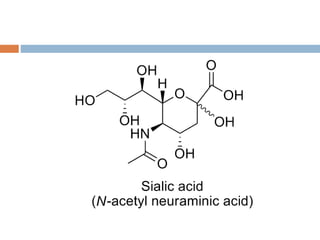
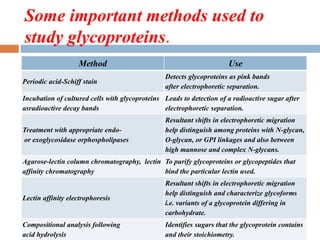
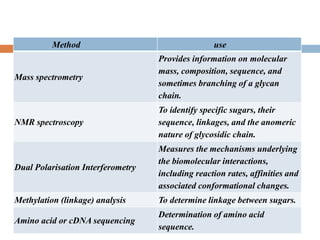
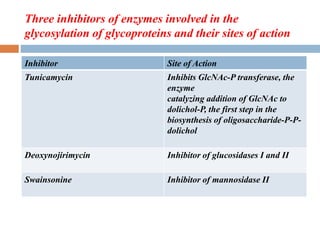
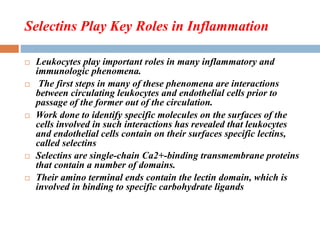
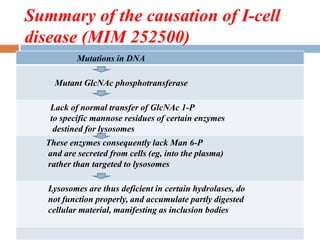
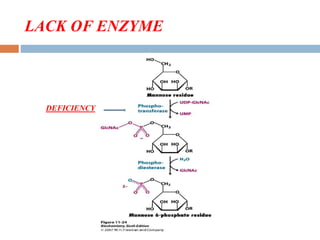
This document provides an introduction to glycobiology and glycoproteins. It defines key terms like glycoproteins, glycosylation, and lectins. It describes the different types of glycoprotein linkages and classes. The roles and functions of glycoproteins are discussed, as well as the sugars commonly found in glycoproteins. Methods for studying glycoproteins like lectins, glycosidases, and mass spectrometry are also summarized.


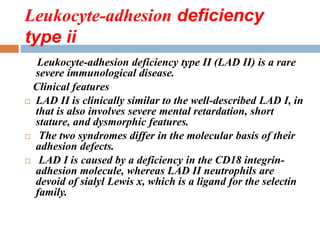
![THERE ARE THREE MAJOR CLASSES
OF GLYCOPROTEINS
Based on the nature of the linkage between their polypeptide chains and
their oligosaccharide chains, glycoproteins can be divided into three
major classes
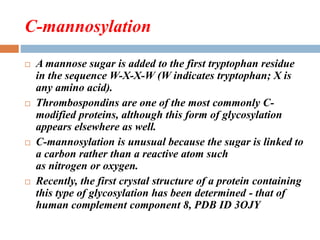
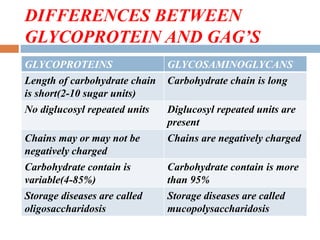
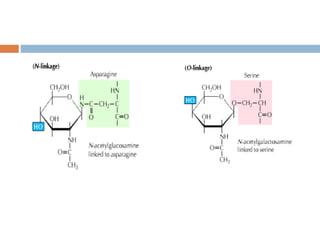
(1) those containing an O-glycosidic linkage (ie, O-linked), involving the
hydroxyl side chain of serine or threonine and a sugar such as N-
acetylgalactosamine (GalNAc-Ser[Thr]);
(2) those containing an N-glycosidic linkage (ie, N-linked), involving the
amide nitrogen of asparagine and N-acetylglucosamine (GlcNAc- Asn);
and
(3) those linked to the carboxyl terminal amino acid of a protein via a
phosphoryl-ethanolamine moiety joined to an oligosaccharide (glycan),
which in turn is linked via glucosamine to phosphatidylinositol (PI). This
latter class is referred to as glycosylphosphatidylinositol- anchored (GPI-
anchored, or GPIlinked) glycoproteins.
Other minor classes of glycoproteins also exist.](https://image.slidesharecdn.com/glycoproteins-150908102816-lva1-app6892/85/Glycoproteins-6-320.jpg)
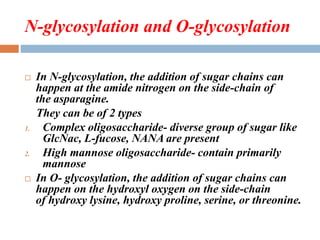

![FEATURES OF N-GLYCOSYLATION
The oligosaccharide Glc3Man9(GIcNAc)2 is transferred from dolichol-
P-P-oligosaccharide in a reaction catalyzed by oligosaccharide:protein
transferase, which is inhibited by tunicamycin.
• Transfer can occur cotranslationally in the endoplasmic reticulum.
• The protein-bound oligosaccharide is then partially processed by
glucosidases and mannosidases; if no additional sugars are added, this
results in a high-mannose chain.
• If processing occurs down to the core heptasaccharide (Man5[GlcNAc]2),
complex chains are synthesized by the addition of GlcNAc, removal of
two Man, and the stepwise addition of individual sugars in reactions
catalyzed by specific transferases (eg, GlcNAc, Gal, NeuAc transferases)
that employ appropriate nucleotide sugars.](https://image.slidesharecdn.com/glycoproteins-150908102816-lva1-app6892/85/Glycoproteins-10-320.jpg)