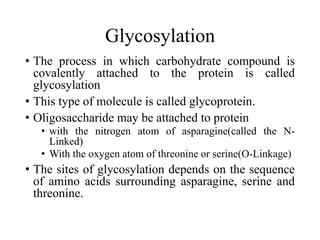
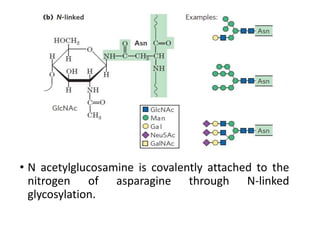
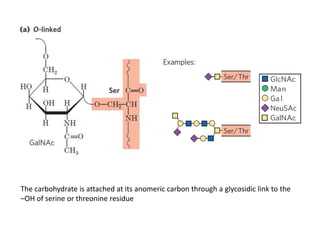
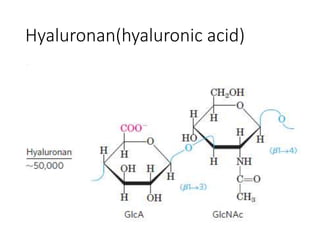
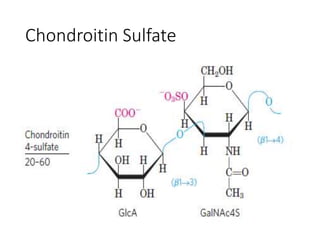
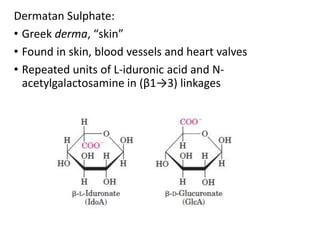
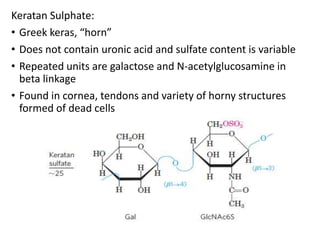
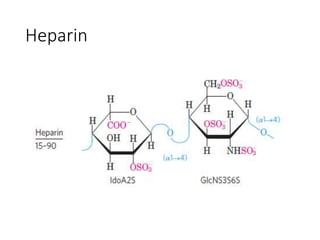
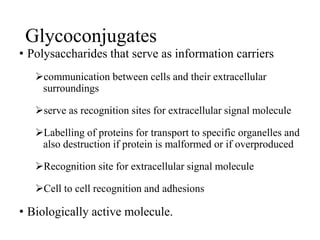
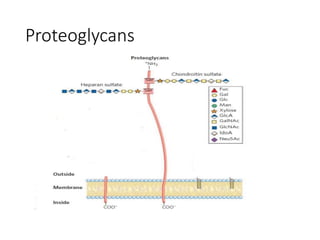
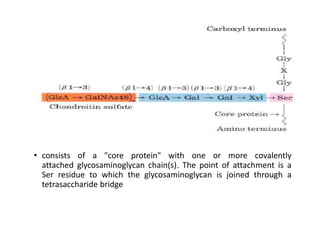
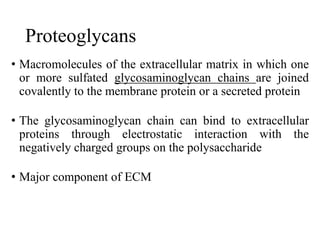
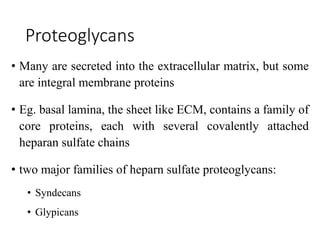
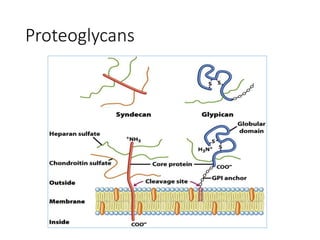
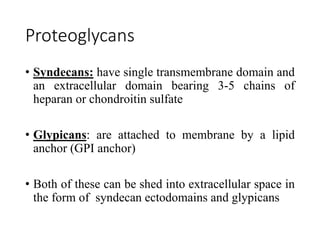
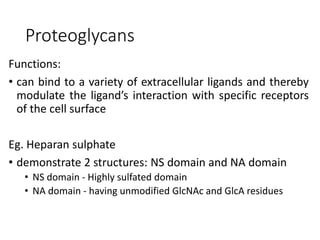
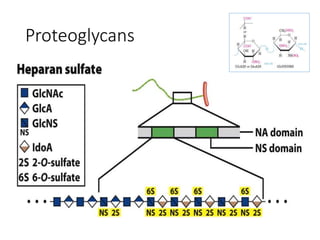
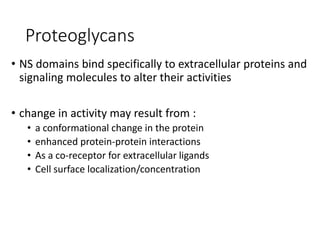
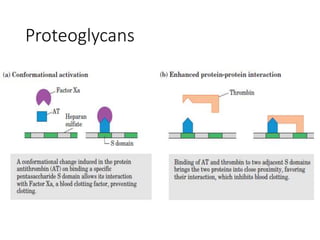
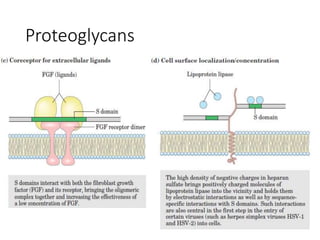
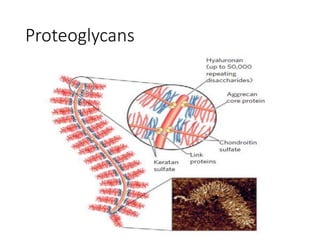
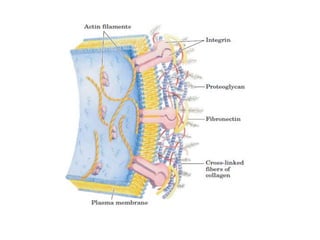
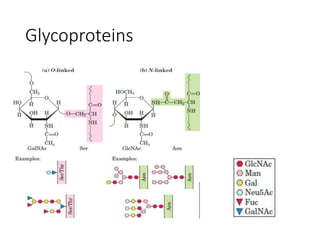
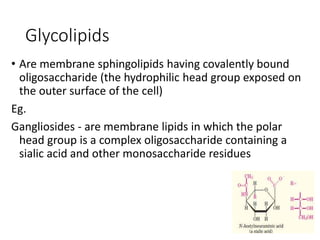
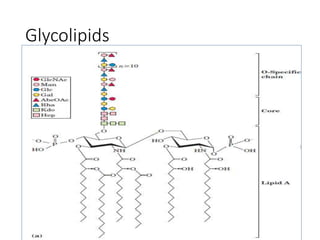
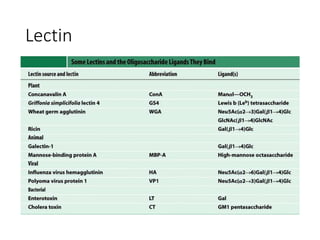
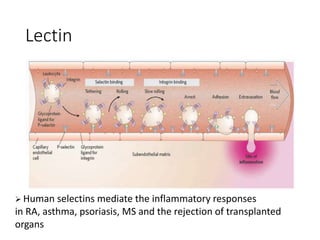
The document discusses carbohydrates and their roles in glycoproteins, glycolipids, and proteoglycans. It explains that carbohydrates are covalently attached to proteins or lipids through glycosylation to form these glycoconjugates. Glycosaminoglycans are linear polysaccharides that help form the extracellular matrix. Common examples discussed include hyaluronic acid, chondroitin sulfate, dermatan sulfate, heparan sulfate, and keratan sulfate. Proteoglycans are core proteins with attached glycosaminoglycan chains that interact with extracellular proteins. Glycoproteins have oligosaccharide chains attached to asparagine or serine/threonine residues. Gly