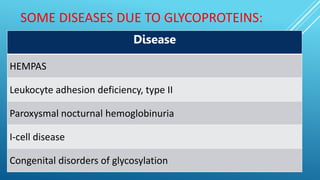
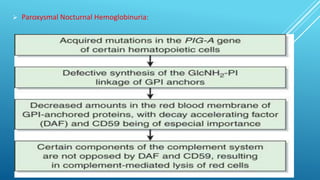
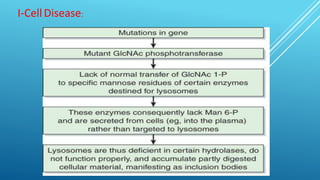
The document discusses glycoproteins, which are proteins with attached oligosaccharide chains, and their biochemical roles, types, and mechanisms of glycosylation. It outlines the differences between O-linked and N-linked glycoproteins, their synthesis, and associated diseases such as Hematopoietic System Dysfunction, Leukocyte Adhesion Deficiency, and Paroxysmal Nocturnal Hemoglobinuria. Additionally, it highlights the impact of glycoproteins on various congenital disorders of glycosylation affecting multiple organ systems.



![ Classes of Glycoproteins:
O-Linked
Glycoproteins
GalNAcSer(Thr)
linkage
GlcNAc-Ser[Thr]
linkage
N-Linked
Glycoproteins
Amide nitrogen of asparagine and Nacetylglucosamine (GlcNAcAsn)
GPI-Linked
Glycoproteins](https://image.slidesharecdn.com/glyco-240110212151-9cc716c2/85/glyco-pptx-5-320.jpg)
![ O-glycosidic linkage-hydroxyl side chain of serine or threonine and a
sugar such as Nacetylgalactosamine (GalNAc-Ser[Thr])
N-glycosidic linkage-amide nitrogen of asparagine and Nacetylglucosamine
(GlcNAcAsn)
Glycosylphosphatidylinositol-anchored
(GPI-anchored, or GPI-linked)- carboxyl terminal amino acid of a
protein via a phosphoryl-ethanolamine moiety joined to an oligosaccharide
(glycan), which in turn is linked via glucosamine to phosphatidylinosito](https://image.slidesharecdn.com/glyco-240110212151-9cc716c2/85/glyco-pptx-6-320.jpg)