The document discusses glycoproteins, detailing their classes, biosynthesis mechanisms, and biological roles, alongside their significance in various diseases. It outlines the differences between O-linked and N-linked glycoproteins, their biosynthetic pathways, and emphasizes the impact of glycoproteins on health, including specific disorders linked to their malfunction. The significance of oligosaccharide chains in modulating glycoprotein properties and their involvement in diseases, such as various congenital disorders and cancer, is also highlighted.


![Classes of Glycoproteins:
O-Linked
Glycoproteins
GalNAcSer(Thr)
linkage
GlcNAc-Ser[Thr]
linkage
N-Linked
Glycoproteins
Complex
Hybrid
High-
mannose
GPI-Linked
Glycoproteins
Other minor
groups](https://image.slidesharecdn.com/glycoproteins-140505233414-phpapp01/85/Glycoproteins-3-320.jpg)











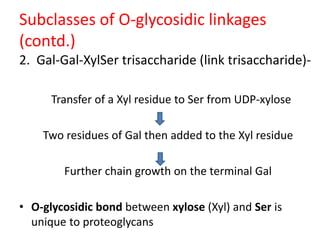

![Subclasses of O-glycosidic linkages
(contd.)
4. GlcNAc-Ser[Thr] linkage –
– On many nuclear proteins and cytosolic proteins.
– a single GlcNAc attached to a serine or threonine
residue.
– formed by donation to Ser (or Thr) of a GalNAc
residue, employing UDP-GalNAc as its donor.](https://image.slidesharecdn.com/glycoproteins-140505233414-phpapp01/85/Glycoproteins-17-320.jpg)




























