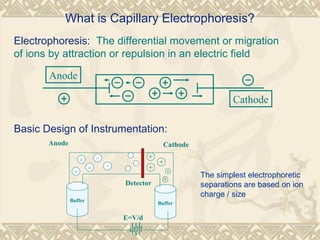
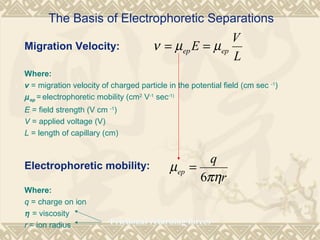
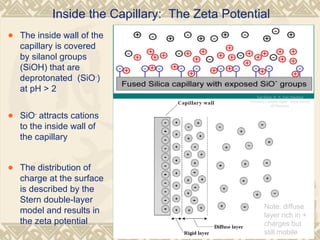
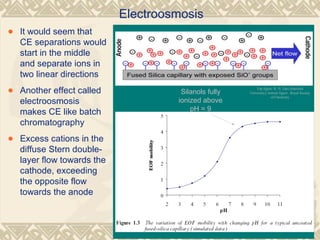
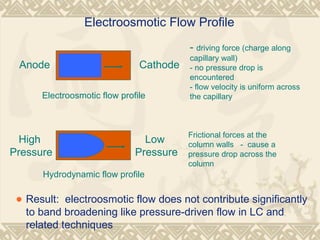
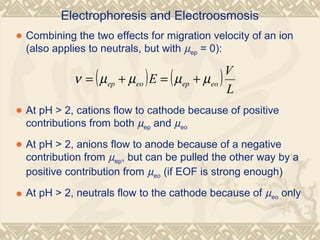
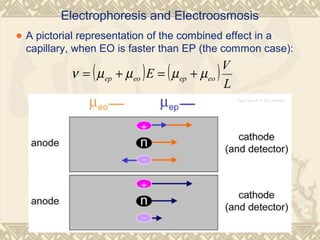
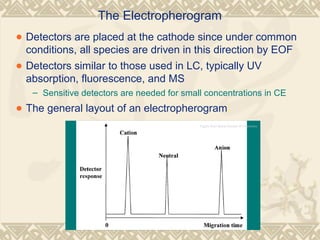
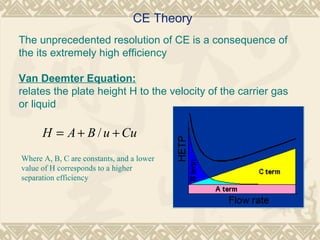
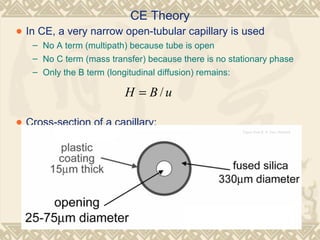
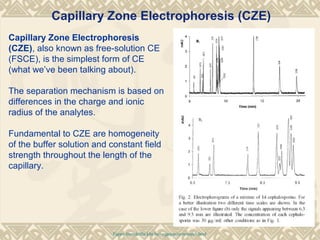
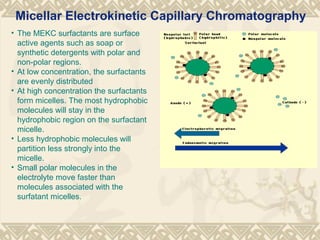
Capillary electrophoresis is a separation technique that uses charged molecules' differential migration in response to an applied electric field. Key components include a capillary, buffers, and detectors. Molecules are separated based on their charge and size. There are several modes, including capillary zone electrophoresis which separates based on charge and size, and micellar electrokinetic capillary chromatography which uses micelles to separate charged and neutral molecules. Capillary electrophoresis provides high resolution, efficiency, and versatility in analyzing various molecules like proteins, nucleic acids, and inorganic ions.