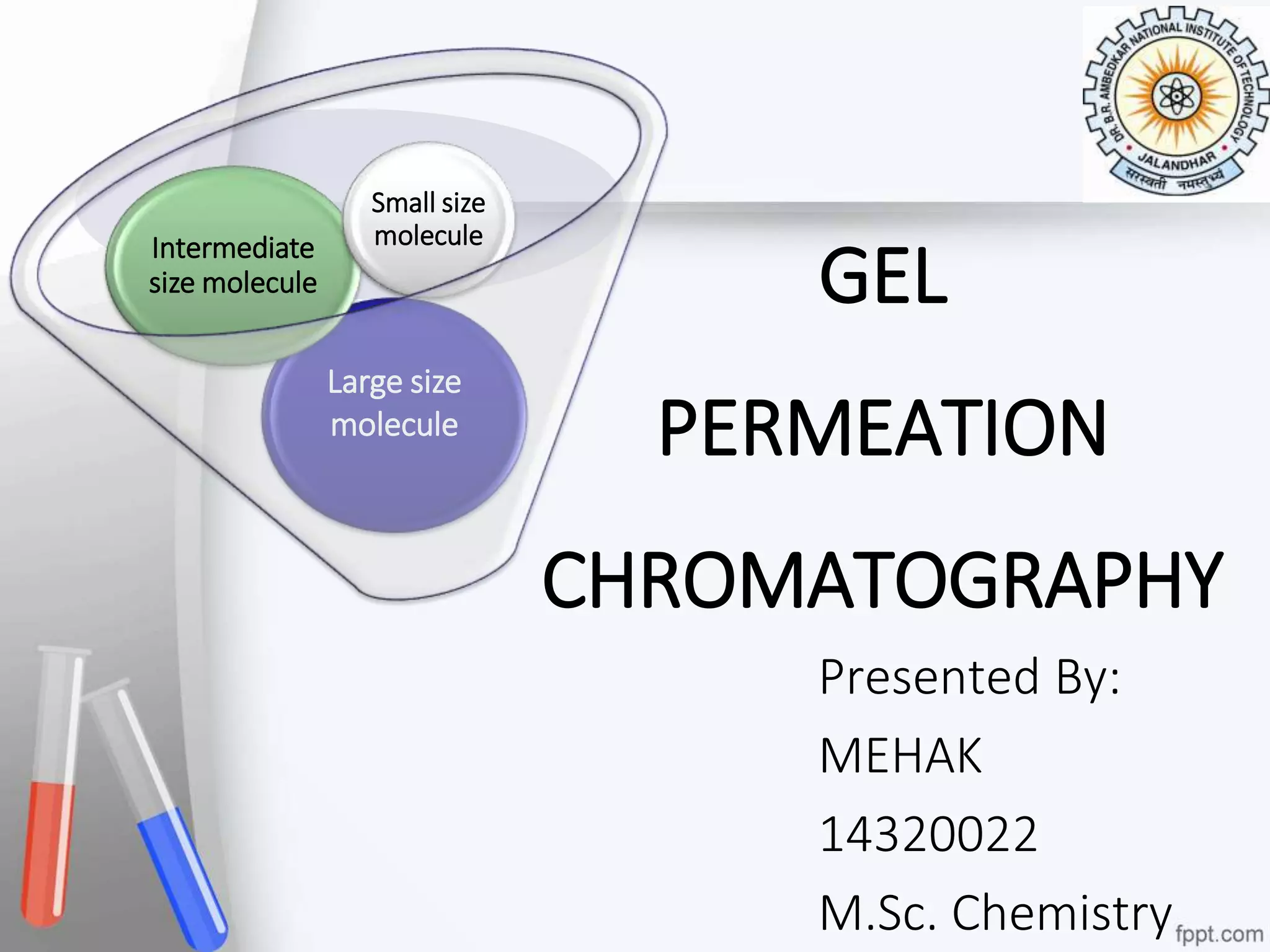
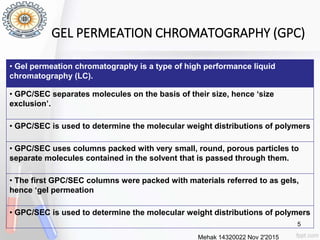
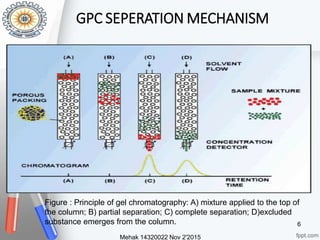
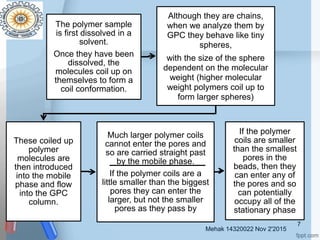
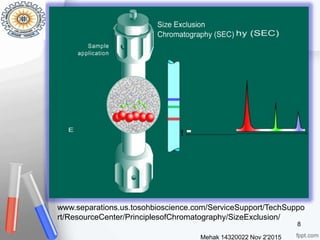
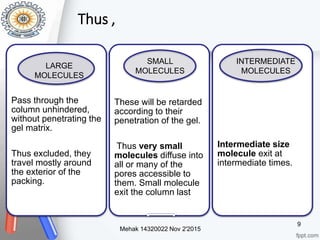
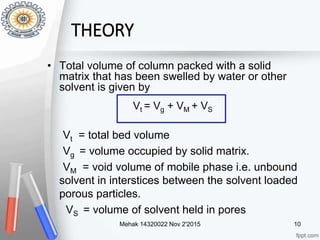
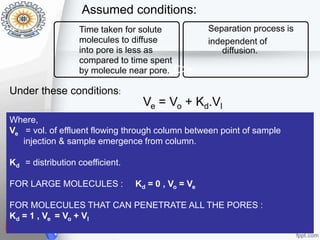
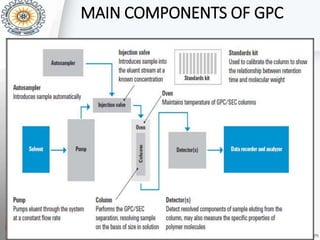
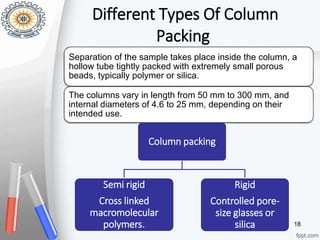
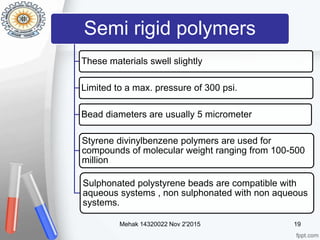
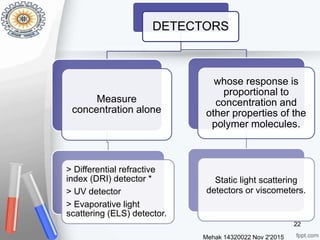
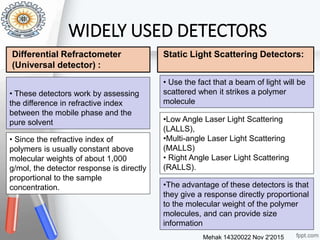
This document provides an overview of gel permeation chromatography (GPC). It begins with definitions of chromatography and discusses the uses and basic mechanism of GPC. The main components of a GPC system are then described, including solvent containers, pumps, columns, detectors, and their purposes. Applications of GPC include determining the molecular weight distributions of polymers like PVC and gum arabic. References for further information on GPC techniques are also provided.