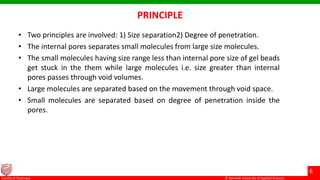
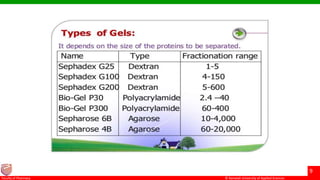
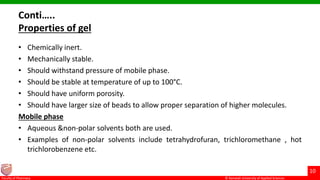
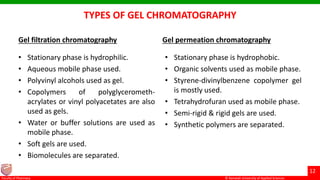
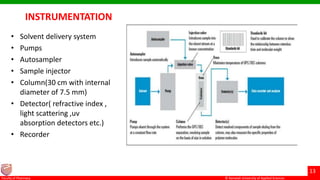
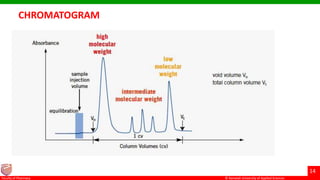
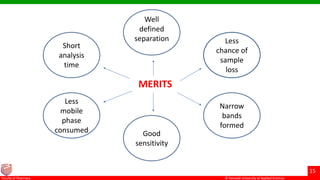
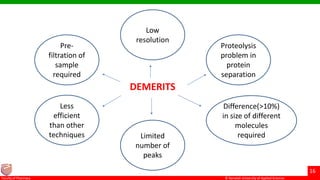
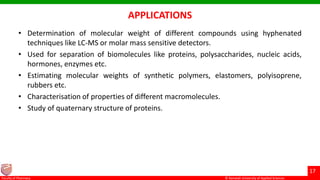
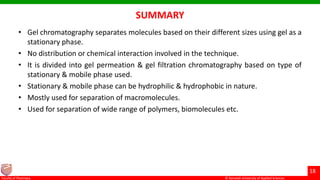
This document discusses gel chromatography, which separates molecules based on size using a porous gel stationary phase. It describes the history, principle, types (gel filtration, gel permeation), instrumentation, applications, and advantages/disadvantages of gel chromatography. The key applications are separation and characterization of biomolecules and synthetic polymers.