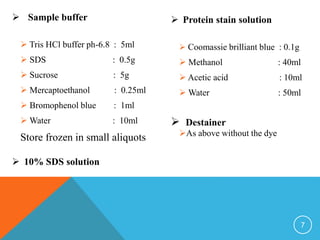
This document provides instructions for performing electrophoresis to separate and characterize proteins. It describes how proteins are linearized and given a negative charge when mixed with SDS detergent, allowing them to be separated by size when an electric current is applied across a polyacrylamide gel medium. Detailed procedures are given for preparing the gel, loading samples mixed with buffer, running the electrophoresis, and staining proteins in the gel for visualization and analysis. A list of standard marker proteins and their molecular weights is also provided for comparison.