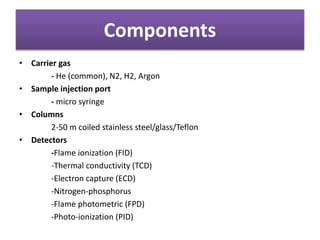
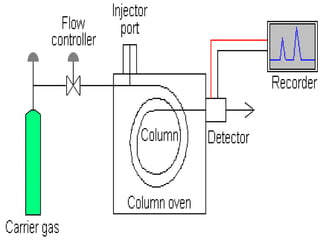
Gas chromatography is a type of chromatography that uses a gaseous mobile phase to separate compounds. It works by partitioning molecules between the gas mobile phase and a liquid stationary phase coated on a solid support. The mixture is vaporized and carried through a column by an inert gas. As the compounds interact differently with the stationary phase, they elute from the column at different times, allowing separation. Key components include the carrier gas, sample injection port, coiled column, and detectors. It has advantages such as applicability to most compounds and sample preservation, while disadvantages include low sensitivity and susceptibility to temperature/flow fluctuations.