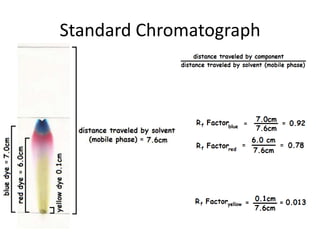
This document summarizes an experiment using paper chromatography to analyze ink samples from four pens (A, B, C, D) and compare them to a sample from a ransom note to determine which pen was used. Paper chromatography works by separating the components in a mixture based on how strongly they interact with the stationary phase (paper) versus the mobile phase (water). Components that interact more strongly with water will travel farther up the paper. By comparing the ink patterns from the pens to the sample from the note, the pen used can be identified.