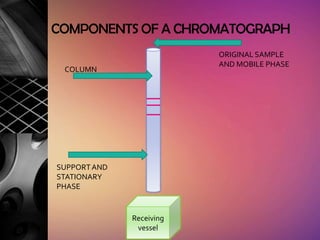
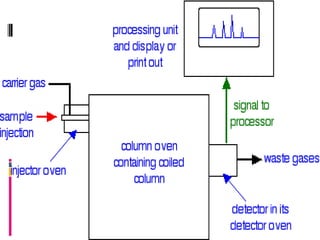
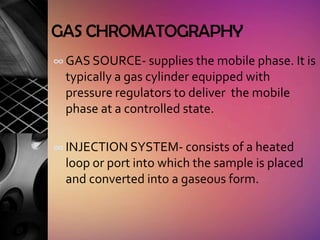
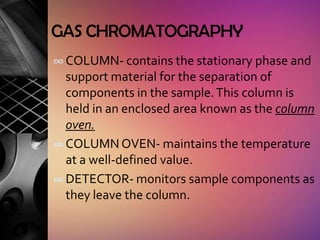
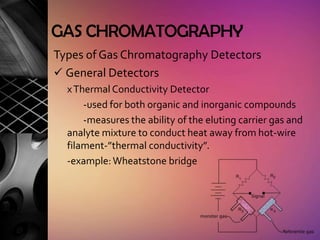
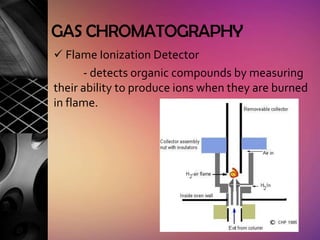
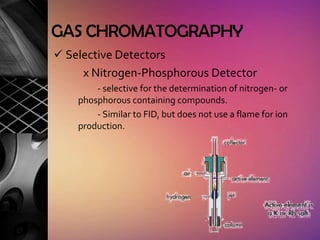
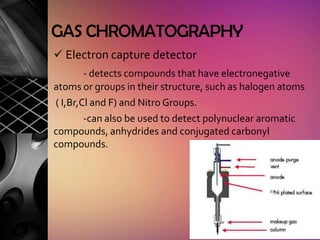
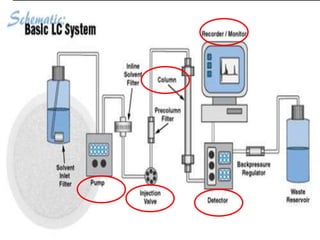
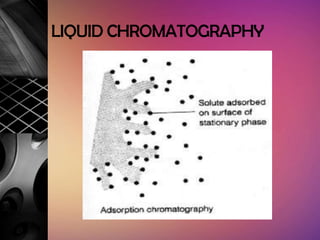
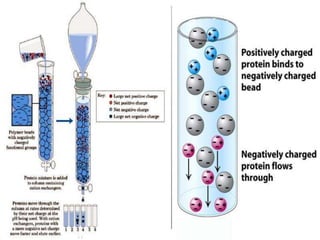
Chromatography is a laboratory technique used to separate mixtures by distributing components between two phases, one stationary and one mobile. The main types are gas chromatography which uses a gas mobile phase, and liquid chromatography which uses a liquid mobile phase. Both work by separating analytes based on how they interact differently with the stationary and mobile phases as they travel through a column. Common applications include analyzing organic compounds, environmental pollutants, and biotechnology samples.