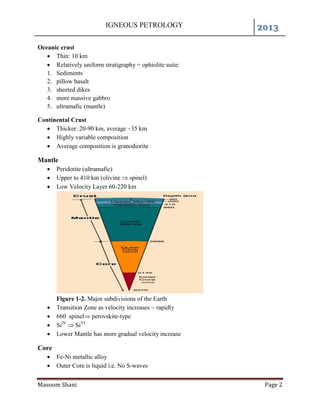
The document provides information on igneous petrology including definitions of key terms like petrography, petrology, and petrogenesis. It describes techniques for classifying igneous rocks based on their texture, mineralogy, chemistry and other properties. Bowen's reaction series is explained as the process by which magma cools and crystallizes into rocks of different compositions. Diagrams like Harker variation diagrams and triangular variation diagrams are used to visualize chemical variations in rock compositions.