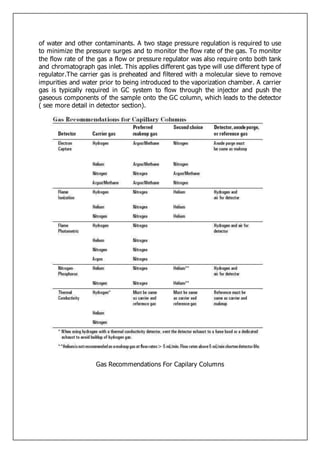
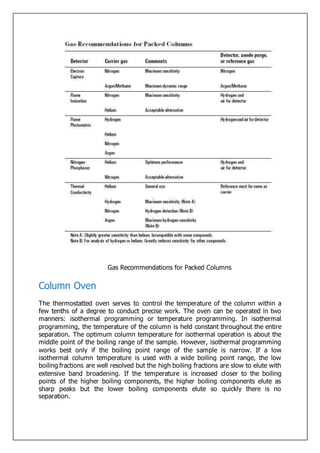
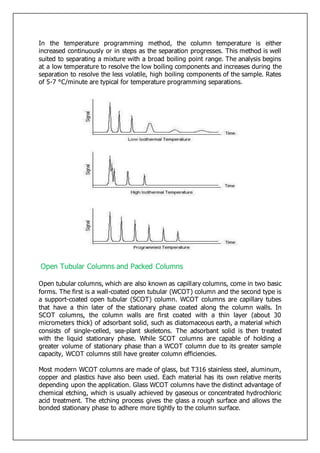
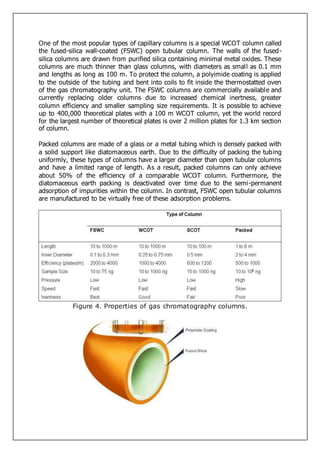
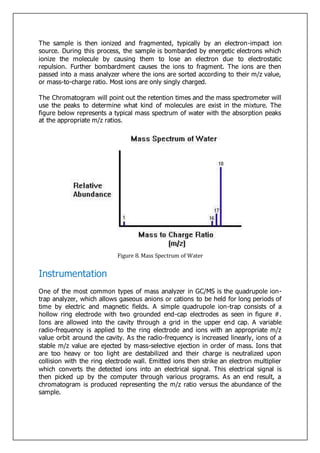
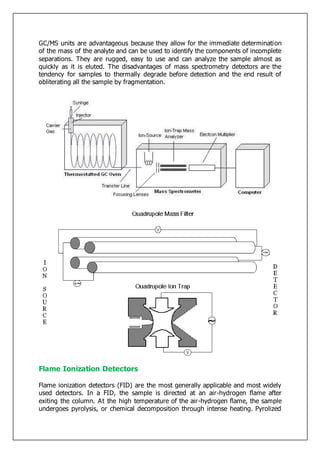
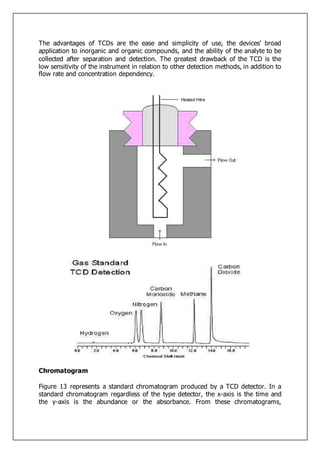
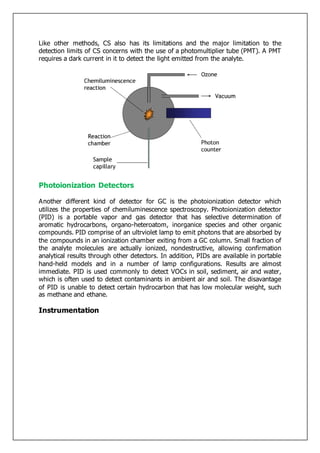
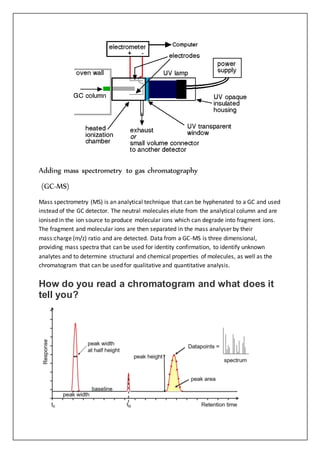
Gas-liquid chromatography separates and detects small molecular weight compounds in the gas phase. The sample is vaporized and swept through a column by an inert carrier gas such as helium. Compounds partition between the stationary and mobile phases, with more polar compounds spending longer in the column and eluting later. The separated compounds are detected and recorded as they exit the column, producing a chromatogram showing peaks of concentration over time. Key components of a gas chromatograph include the carrier gas supply, sample injection system, separation column coated with a stationary phase, and detector.