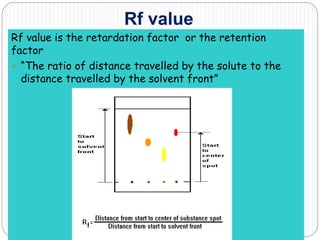
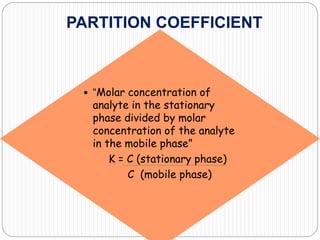
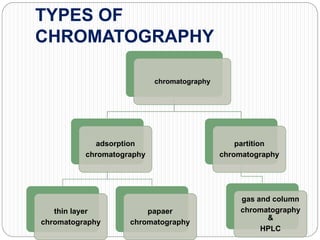
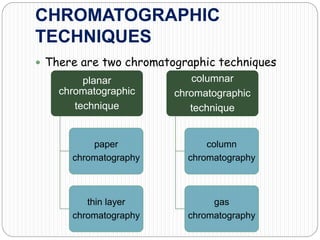
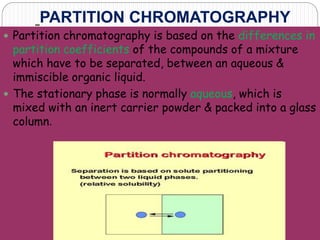
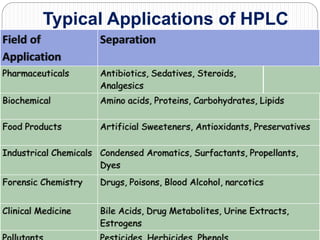
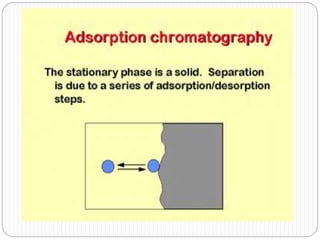
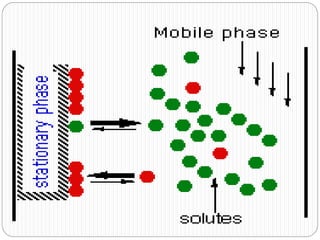
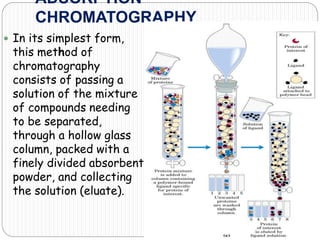
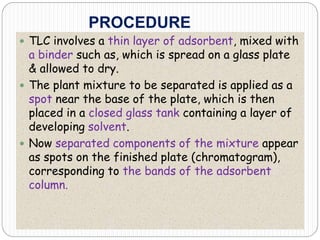
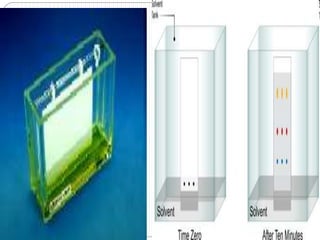
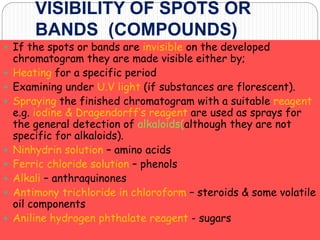
This document discusses the use of chromatography techniques to separate plant constituents. It begins with background on pharmacognosy and why extraction of therapeutic vs non-therapeutic constituents is important. Chromatography techniques like thin layer chromatography, gas chromatography, and HPLC are described for separating mixtures based on differences in compounds' affinities for mobile vs stationary phases. Specific discussion is provided on separating alkaloids, volatile oils, and sugars using appropriate solvent systems and chromatographic conditions.