The document summarizes several gas laws:
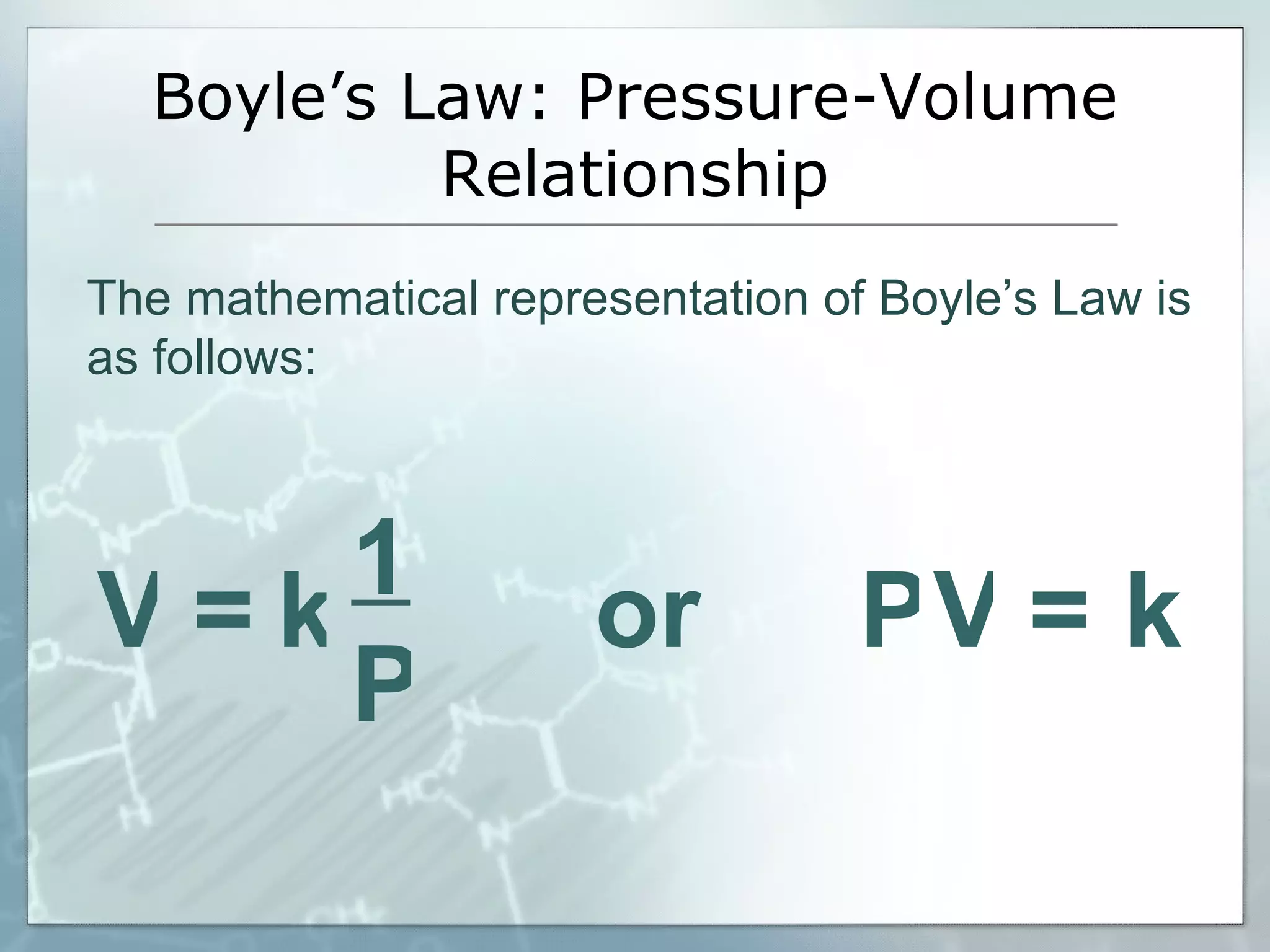
- Boyle's law relates the inverse relationship between gas volume and pressure at constant temperature
- Charles' law describes how gas volume increases with temperature at constant pressure
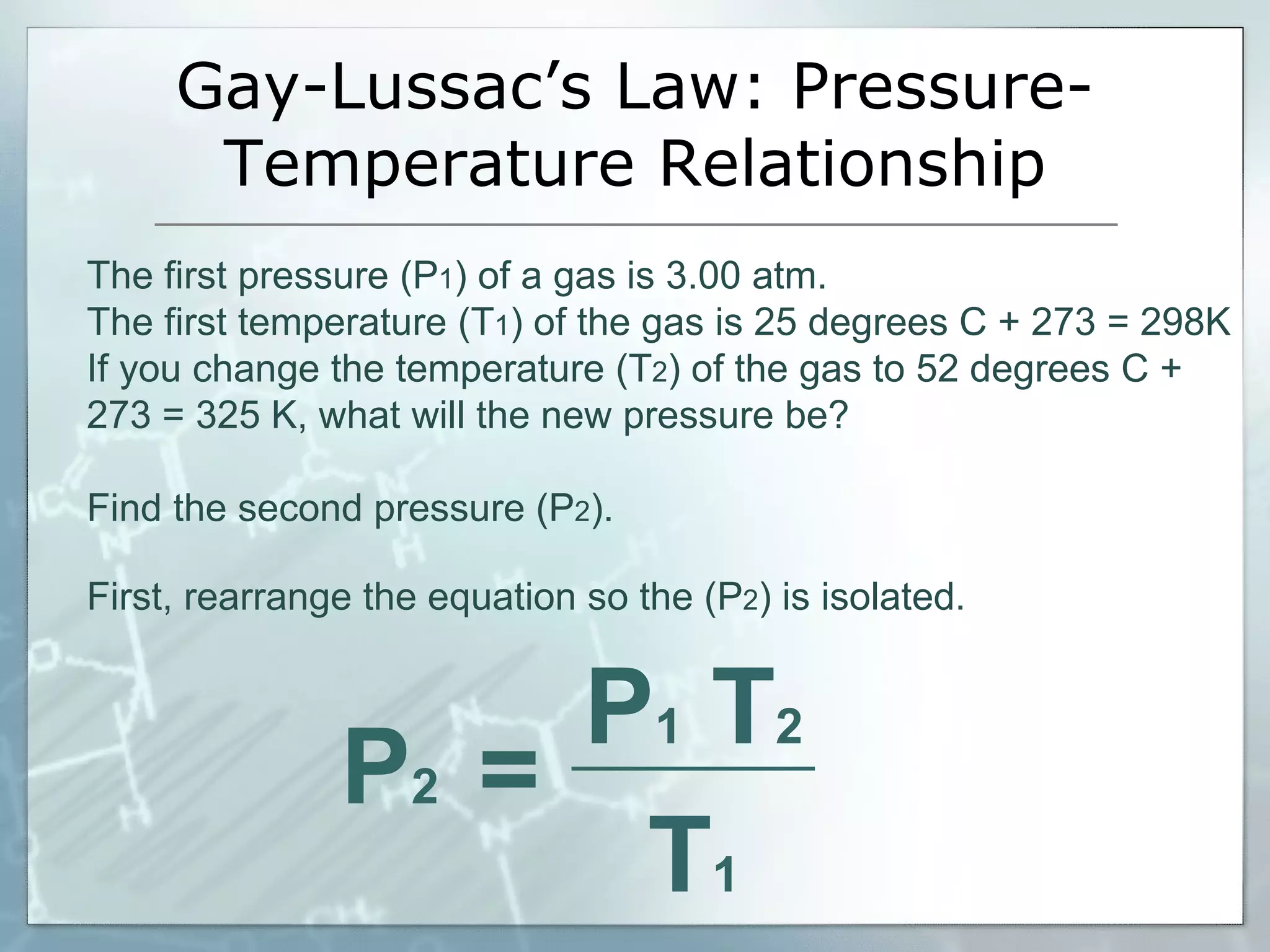
- Gay-Lussac's law explains how gas pressure rises with increasing temperature at constant volume
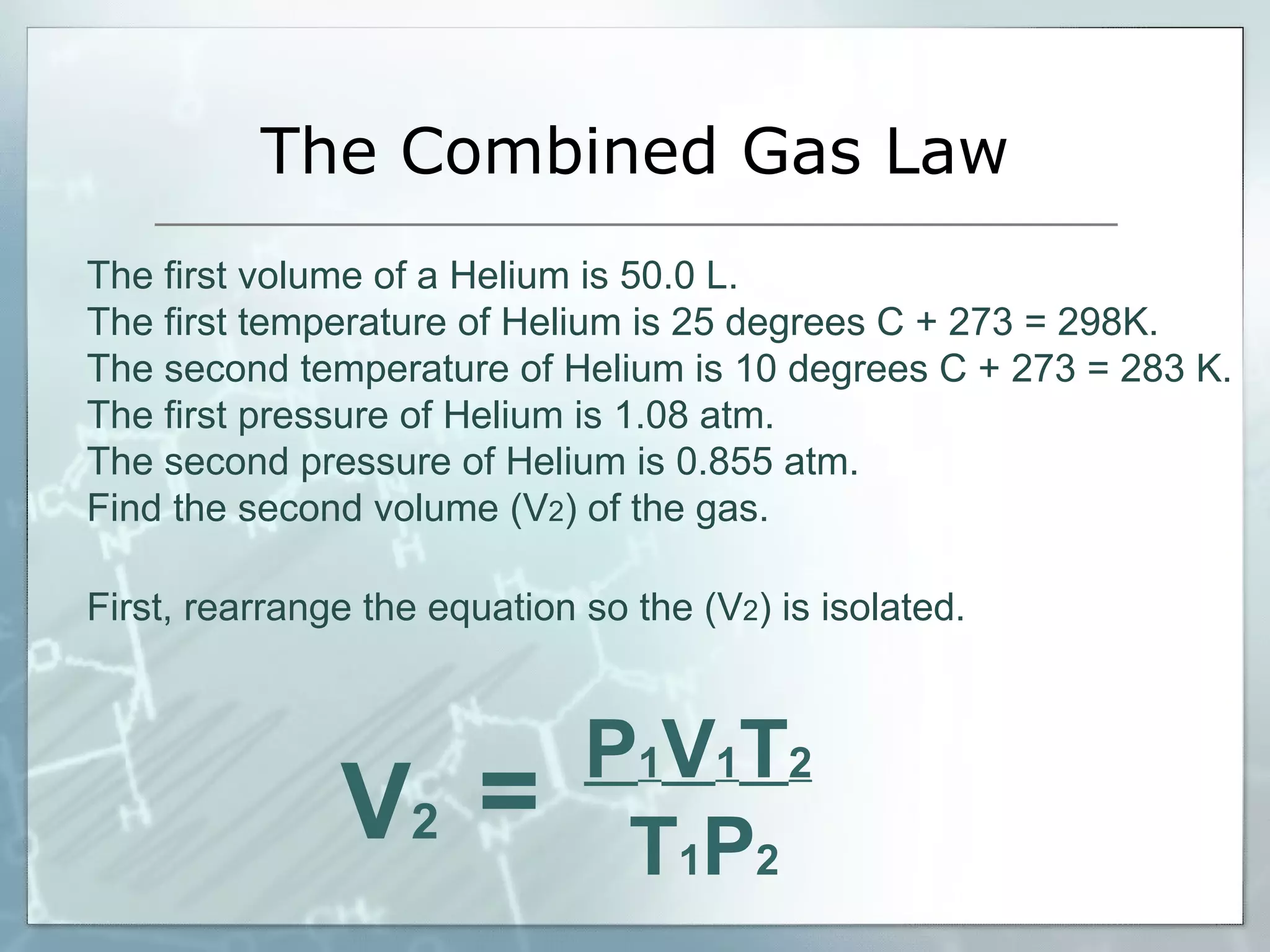
- Combined gas law incorporates changes in pressure, volume, and temperature for a fixed amount of gas
- Dalton's law of partial pressures states that the total pressure of a gas mixture equals the sum of the individual gas pressures