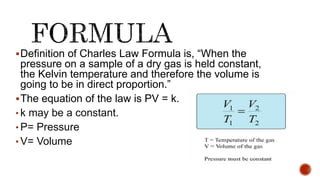
Charles' law describes how gas volume changes with temperature. It states that the volume of a gas is directly proportional to its temperature when pressure is kept constant. The document provides the formula for Charles' law and shows examples of using it to calculate unknown volumes or temperatures given other variables like initial and final volumes and temperatures. It also discusses the limitations of Charles' law and provides sample problems and solutions demonstrating how to apply the law to calculate unknown values.