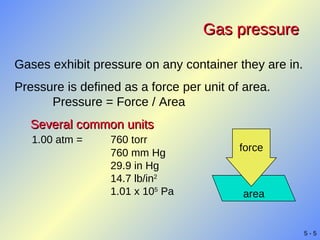
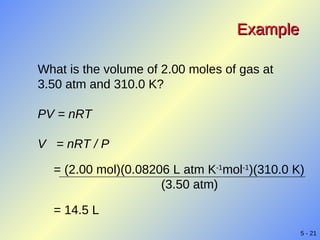
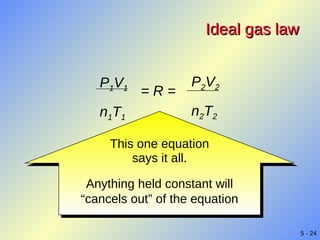
- Gases exhibit behaviors described by gas laws such as Boyle's law, Charles' law, and Gay-Lussac's law. The ideal gas law combines several gas laws into a single equation relating pressure, volume, temperature, and moles of gas.
- Real gases deviate from ideal gas behavior due to intermolecular forces and molecular size. The van der Waals equation accounts for these factors.
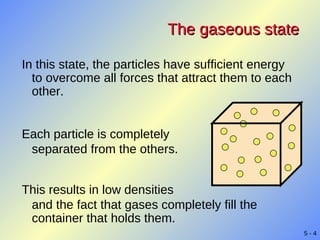
- Kinetic molecular theory explains gas properties in terms of the high-speed motion and infrequent collisions of molecules separated by large distances.