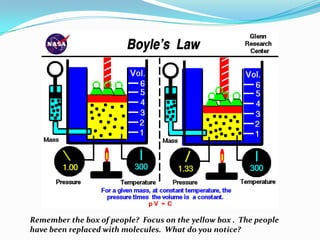
Boyle's Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. It states that if temperature is held constant, the pressure of a gas varies inversely with its volume. The document provides examples of how Boyle's Law applies to scuba diving and calculating gas pressure and volume changes. It also explains how to use the equation PV=kT and P1V1=P2V2 to calculate pressure and volume relationships for gases.