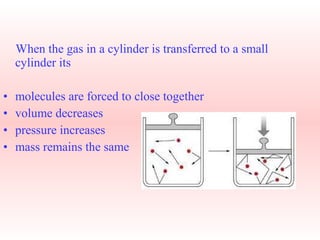
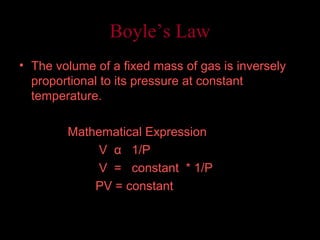
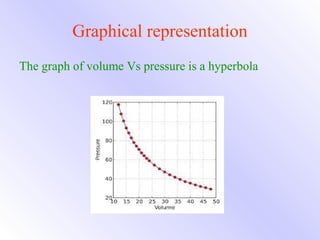
This document explains Boyle's law, which states that the volume of a gas varies inversely with pressure when temperature is kept constant. It provides examples of how Boyle's law applies to balloons expanding with decreasing pressure, and gas compressing into a smaller volume under increasing pressure. Mathematical and graphical representations of the inverse pressure-volume relationship are also presented.