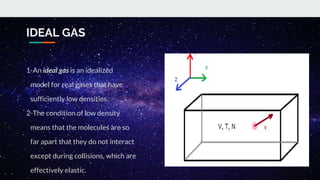
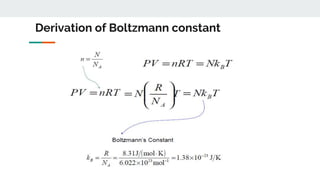
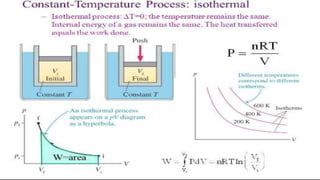
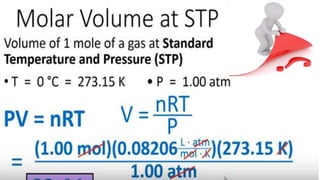
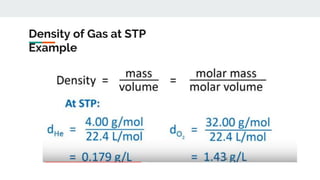
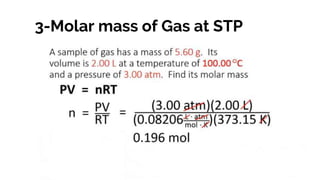
The document covers the history and principles of ideal gases, including Avogadro's number, the ideal gas law, and real gas behavior. It explains key concepts such as the relationship between pressure, volume, and temperature, alongside real-life applications of gas laws, such as in safety and efficiency. Additionally, it discusses notable contributions from figures like Emile Clapeyron and Boltzmann in thermodynamics and atomic theory.