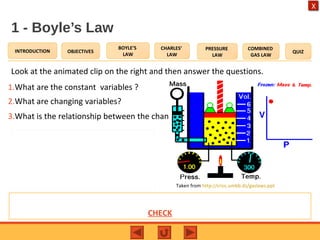
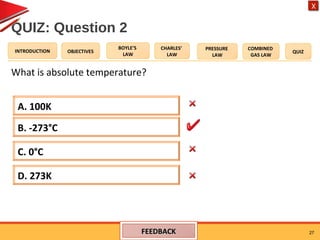
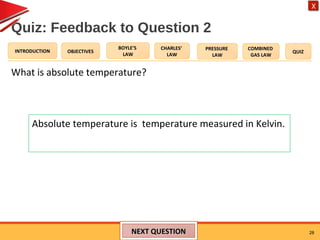
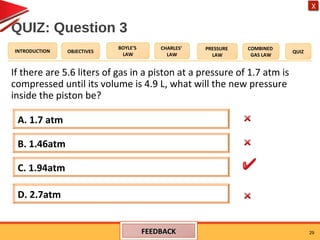
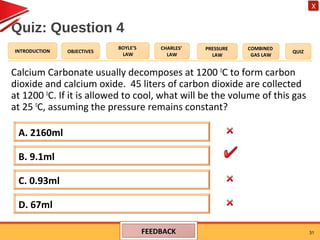
The document provides an introduction to gas laws including Boyle's law, Charles' law, the pressure law, and the combined gas law. It includes objectives, demonstrations, examples, graphs, and a quiz. The key points covered are:
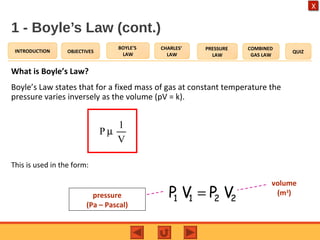
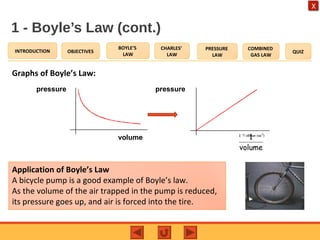
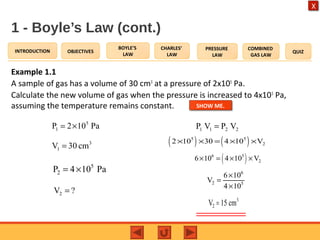
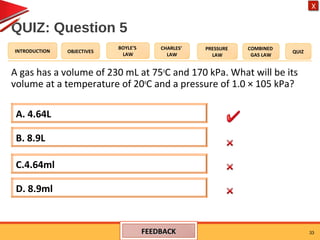
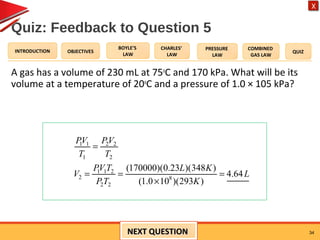
- Boyle's law states that the pressure and volume of a gas are inversely proportional at constant temperature.
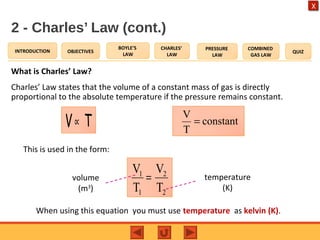
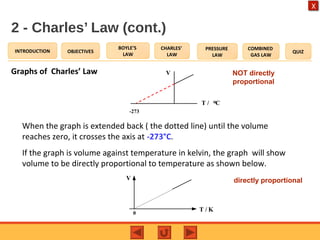
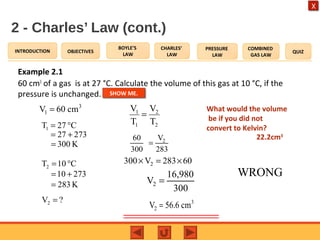
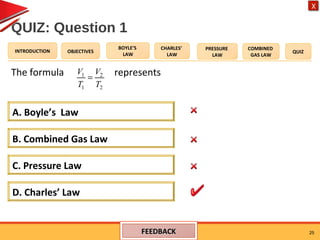
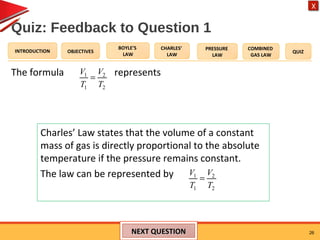
- Charles' law states that the volume of a gas is directly proportional to its temperature when pressure remains constant.
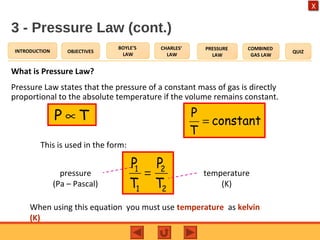
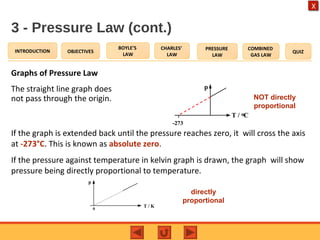
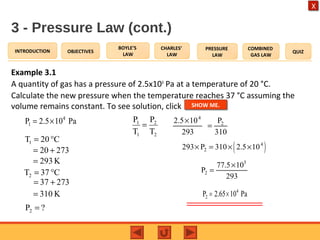
- The pressure law states that the pressure of a gas is directly proportional to its temperature at constant volume.
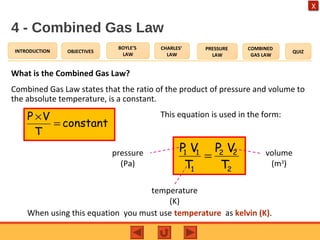
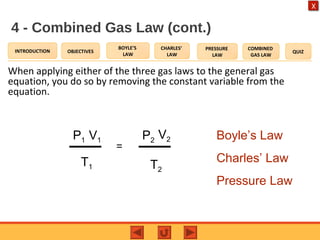
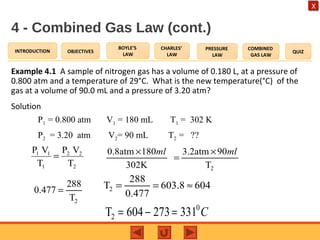
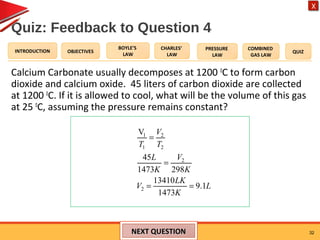
- The combined gas law relates the pressure, volume, and temperature of a gas, stating their product over temperature is a constant.