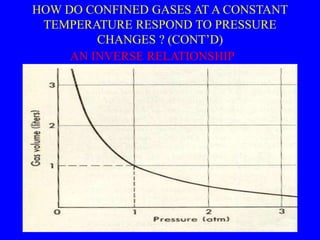
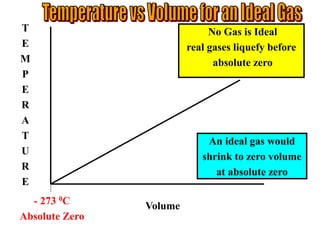
This document discusses the properties of gases and the gas laws. It explains that gases have higher energy, lower density, and flow to fill their container compared to liquids and solids. The document also describes how temperature and pressure are measured, noting that pressure is measured using a barometer that reads the height of mercury. It then explains the gas laws of Boyle's Law and Charles' Law, which describe how the volume of a gas changes with pressure and temperature respectively when other variables are held constant.