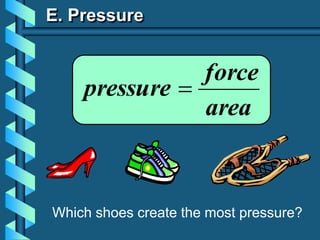
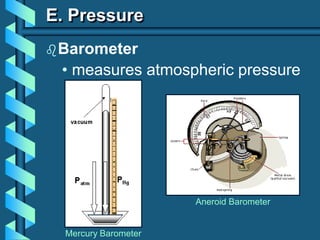
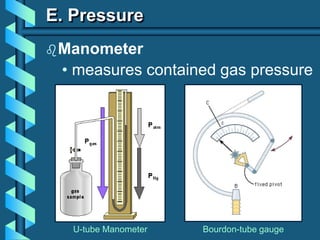
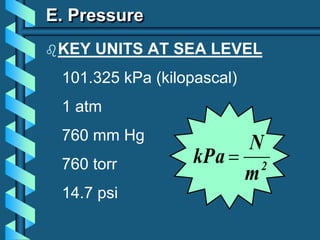
The document describes several key concepts relating to gases:
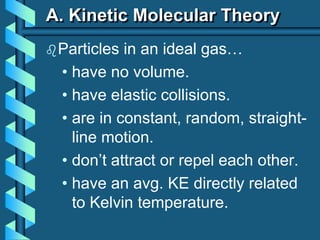
1) It outlines the postulates of kinetic molecular theory and how they describe the behavior of ideal gases.
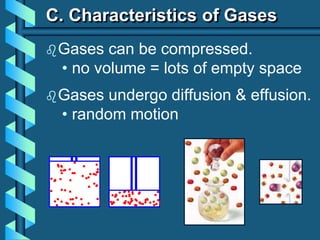
2) It then discusses how real gases differ from ideal gases and how their behavior is affected by factors like pressure and temperature.
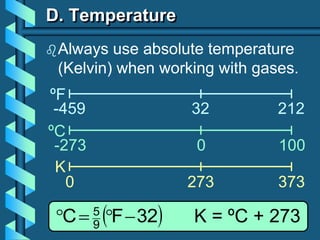
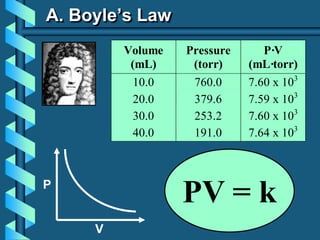
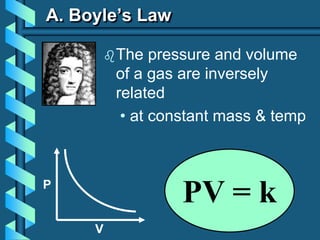
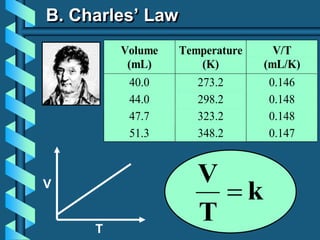
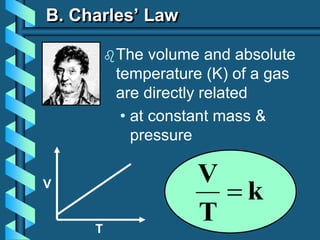
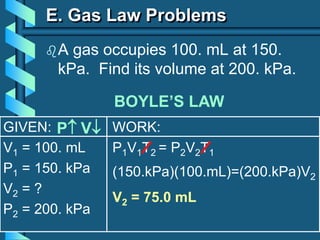
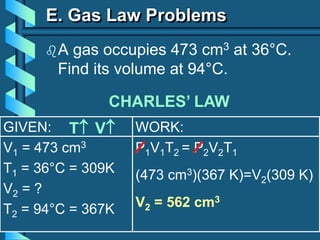
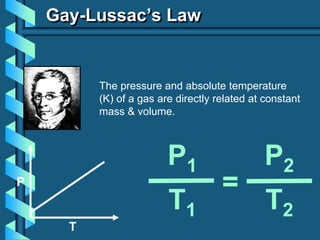
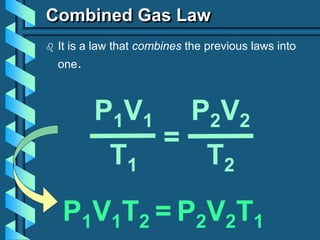
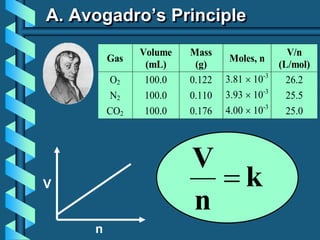
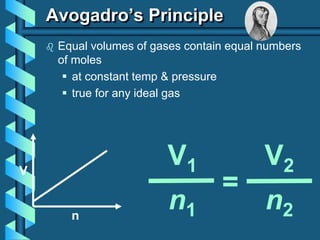
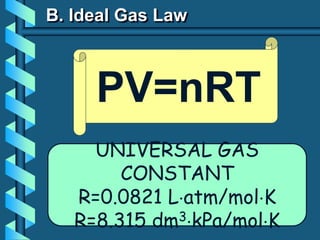
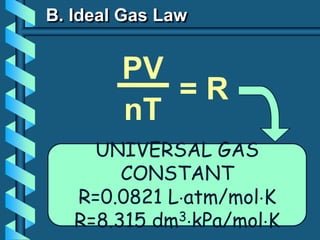
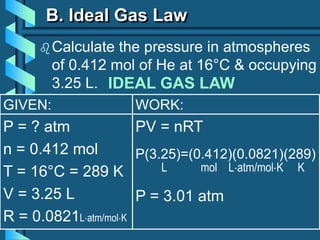
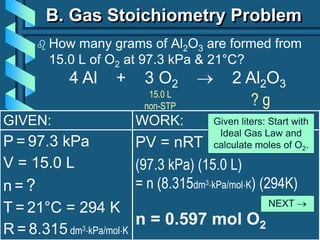
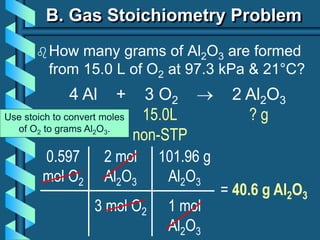
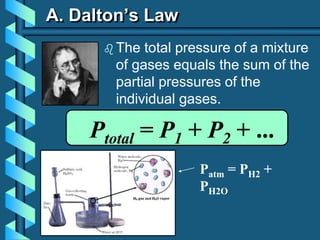
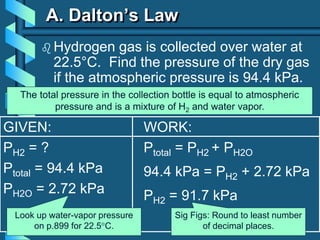
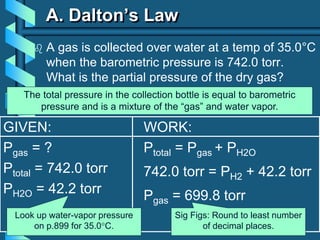
3) Several gas laws are introduced that describe the relationships between pressure, volume, temperature, and number of moles for gases including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, Dalton's law of partial pressures, and the ideal gas law.