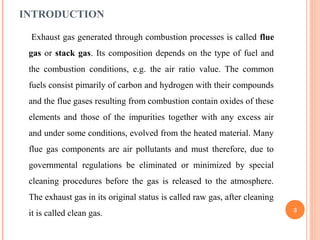
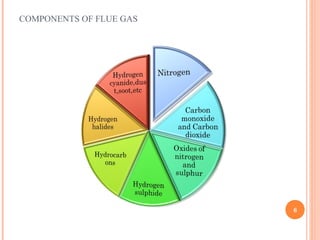
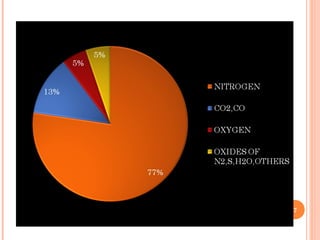
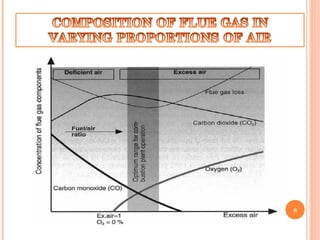
Flue gas, or exhaust gas, is generated through combustion processes. It contains oxides of carbon, hydrogen, and other elements from the fuel, along with any excess air. Many components are air pollutants that must be cleaned or minimized before release. Flue gas analysis indicates the combustion efficiency and air-to-fuel ratio. It can be used to predict flue sizes and losses. Common analysis techniques include gas chromatography, mass spectroscopy, and indicators that detect specific components like carbon monoxide. Proper flue gas analysis promotes safety, efficiency, and process optimization.








![References :
[1] Flue gas analysis, Wikipedia
[2] Products of combustion of gaseous fuels, by
Edwin L. Hall ARA Lab.
[3] North American combustion handbook, by the
North American Manufacturing Company, Ohio
[4] Gas Liquid chromatography, by Stephen Dal
[5] Modern Gas Analysis, by P.W.Mullen
[6] Manual for Hydrocarbon Analysis, by AATM
Special Technology Publisher No. 332
21](https://image.slidesharecdn.com/fluegasanalysis-140823084350-phpapp02/85/Flue-gas-analysis-21-320.jpg)

