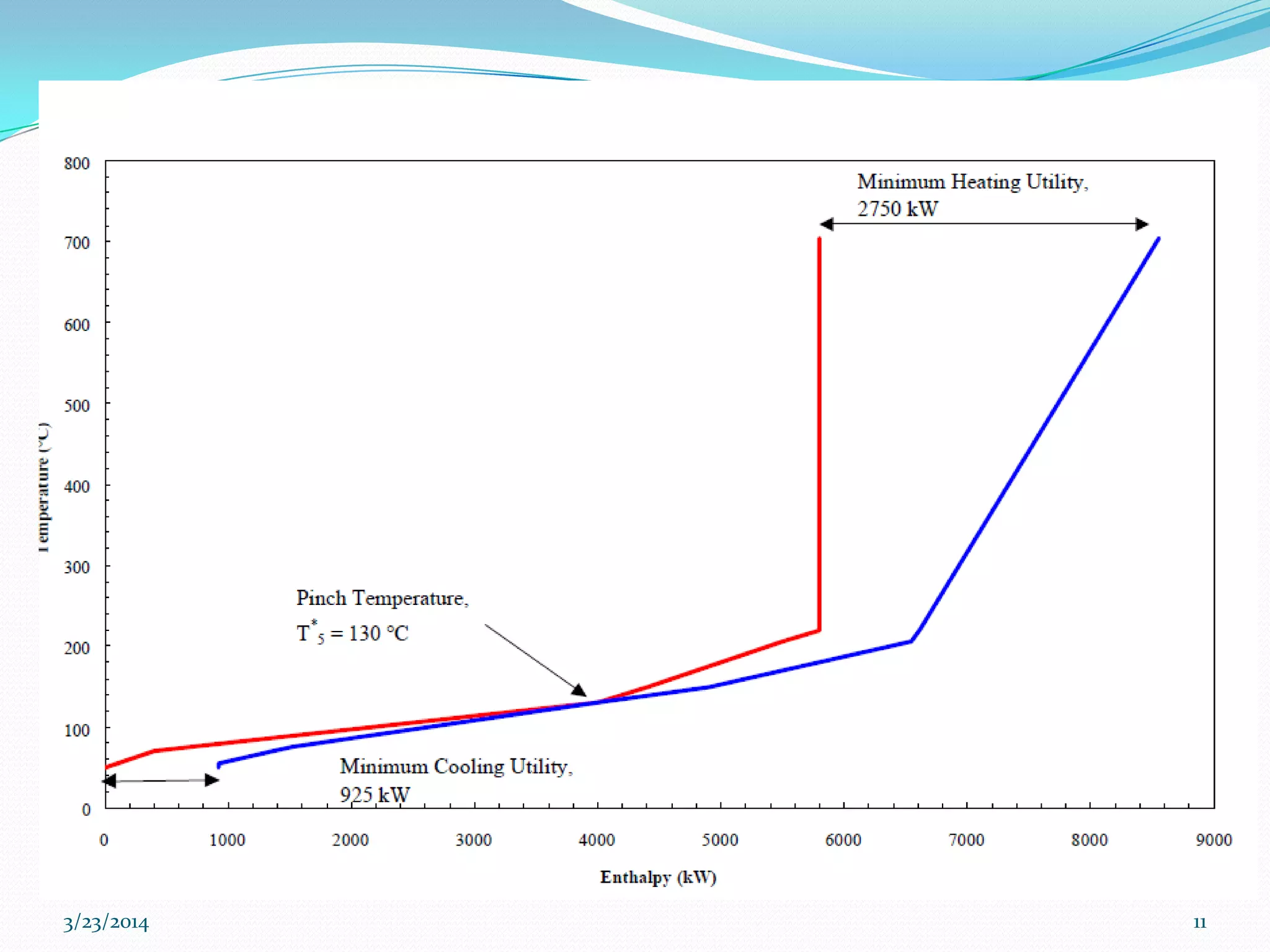
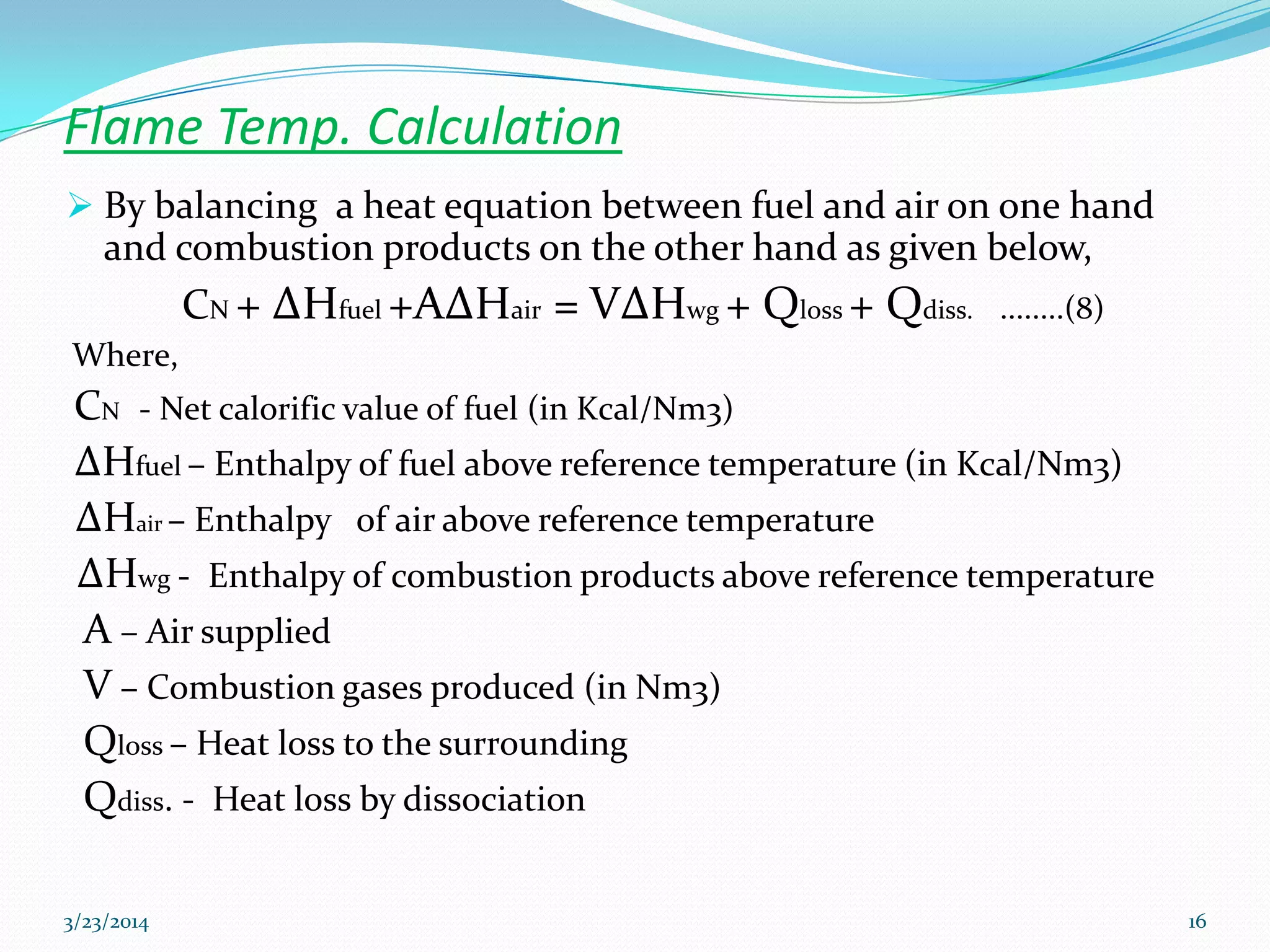
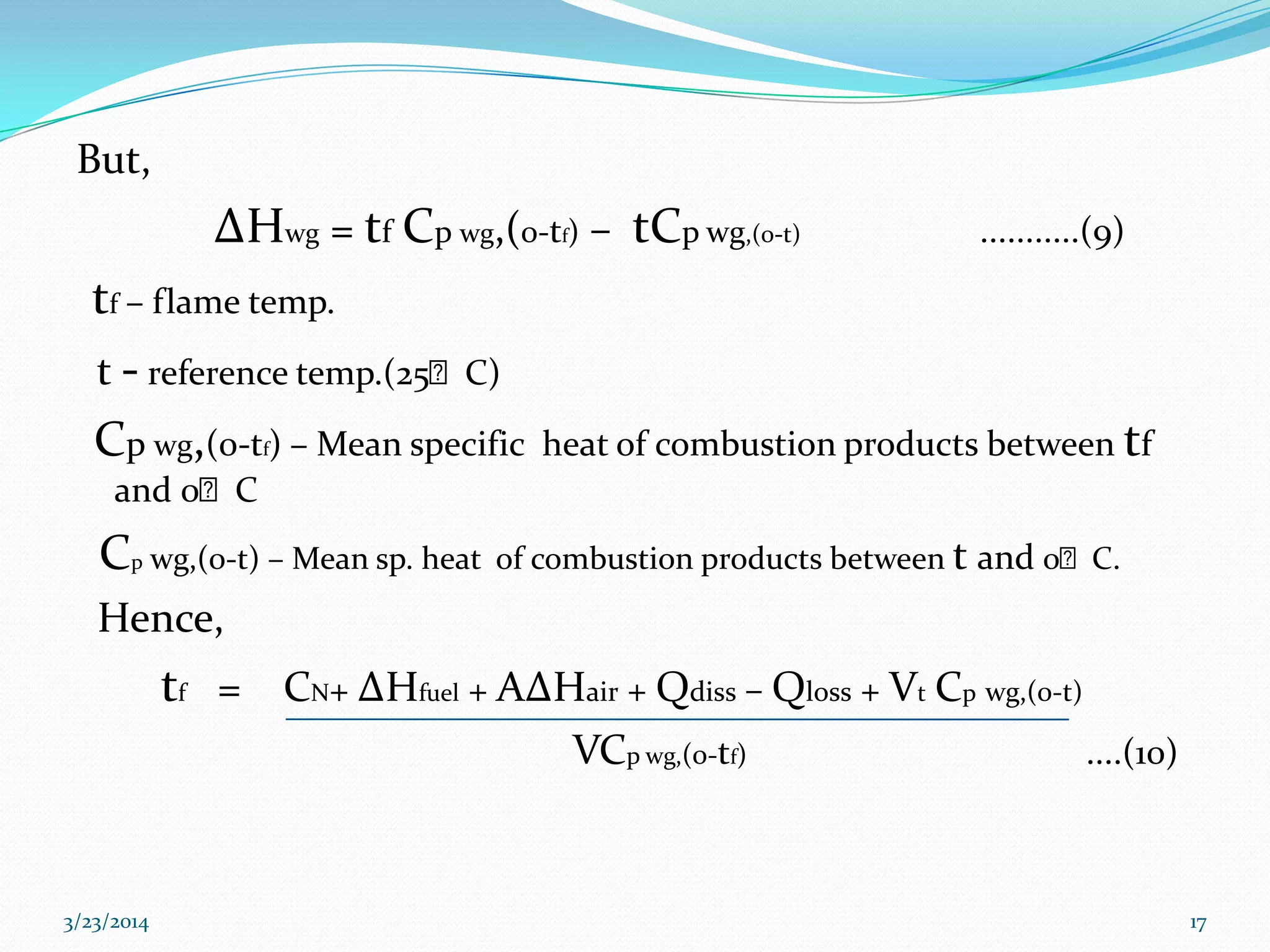
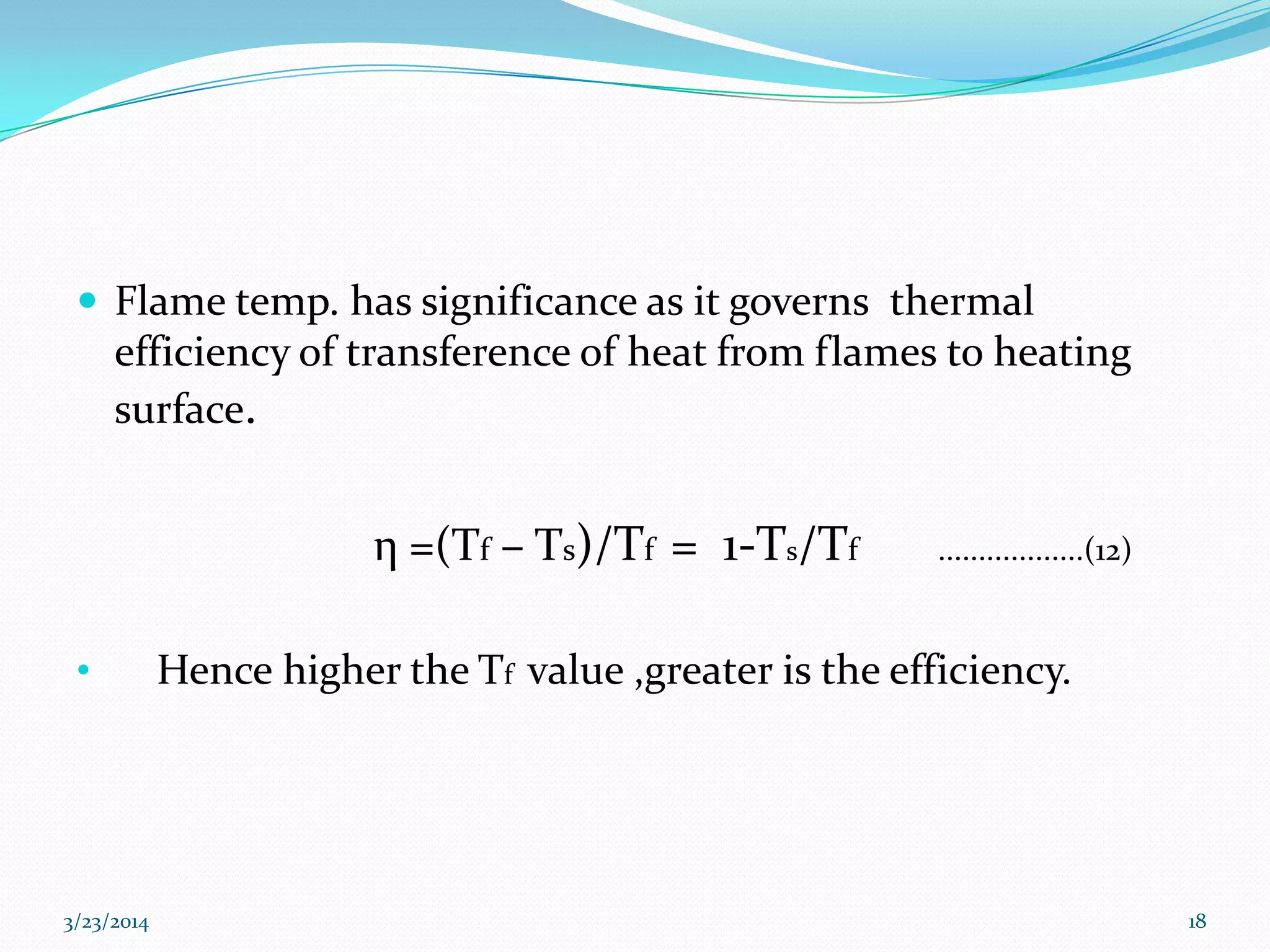
This document discusses key thermodynamic concepts related to combustion processes, including:
1) Heat of combustion, flame temperature, enthalpy of combustion systems, and equilibrium constants of combustion reactions are the major thermodynamic functions that influence fuel utilization.
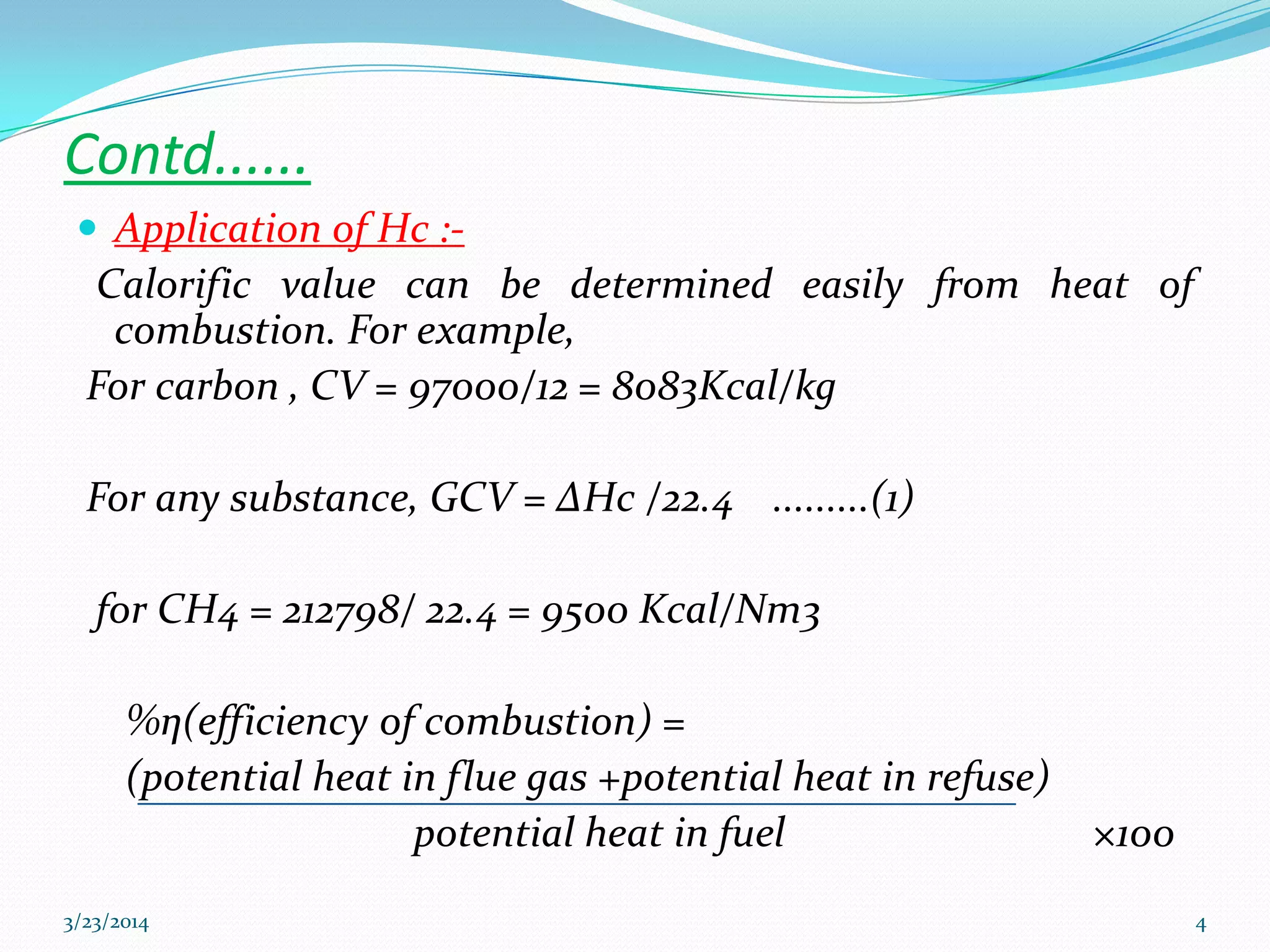
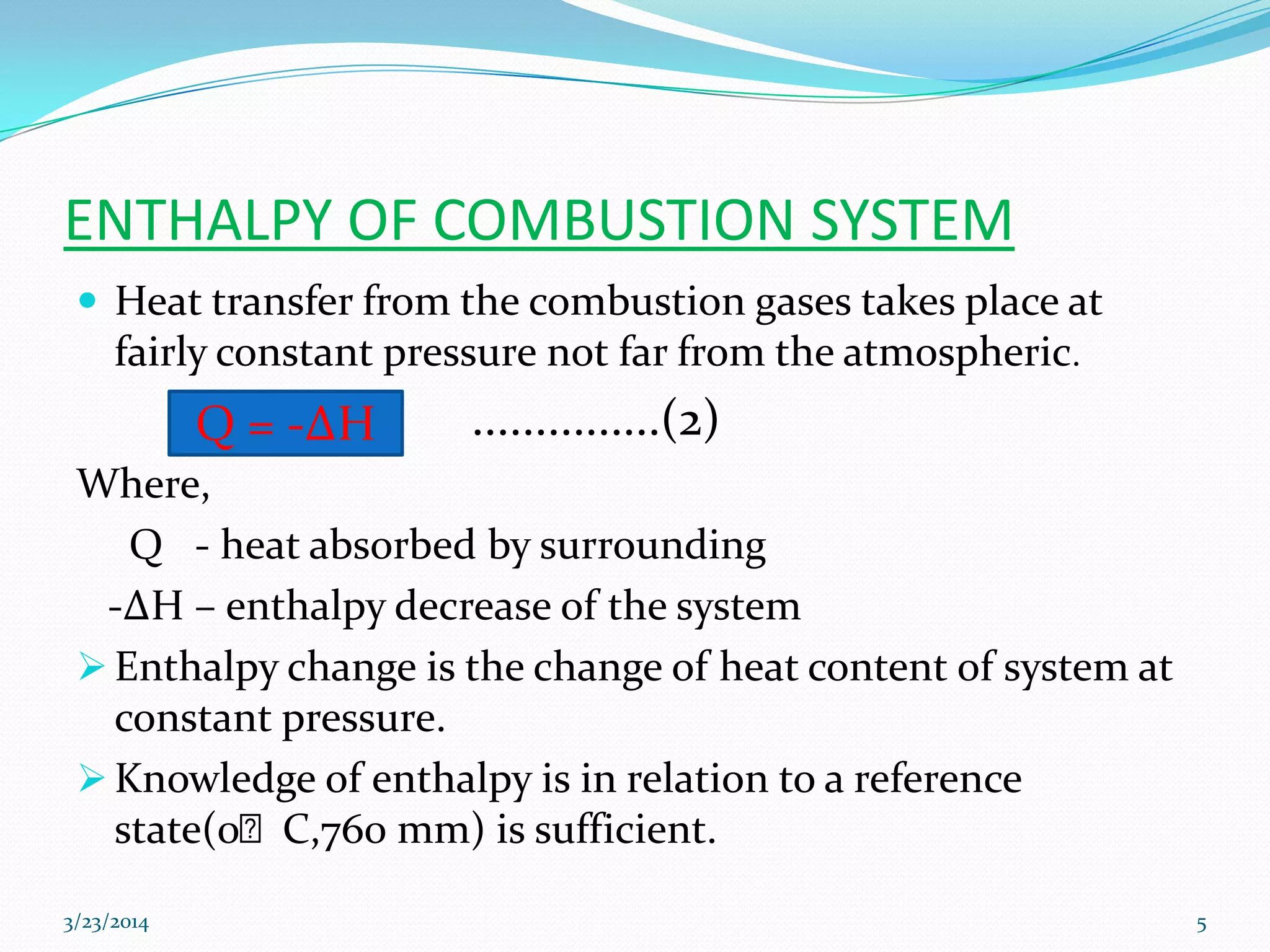
2) Heat of combustion represents the potential heat of a fuel and can be used to calculate calorific value. Enthalpy is the heat content of a system at constant pressure.
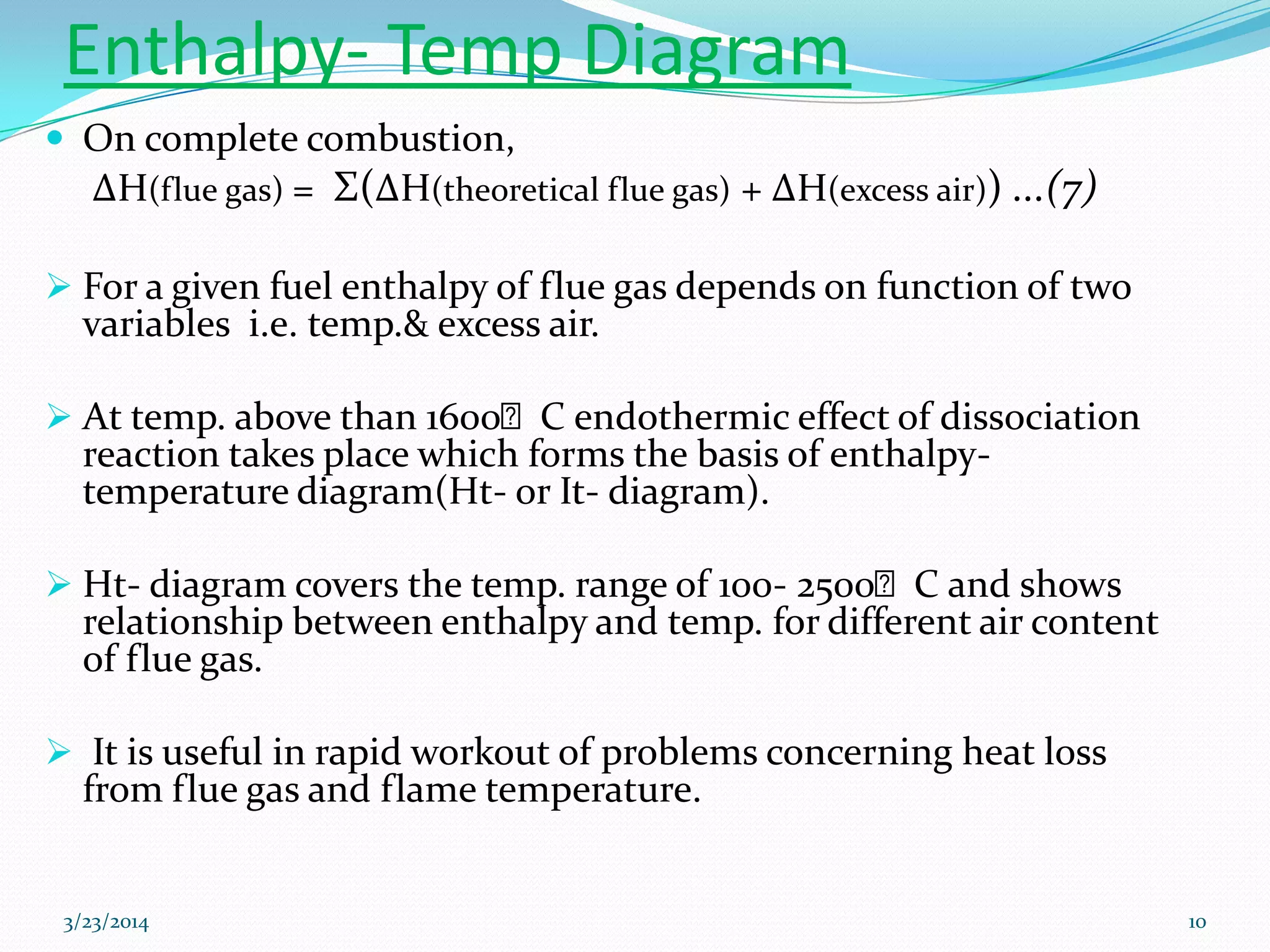
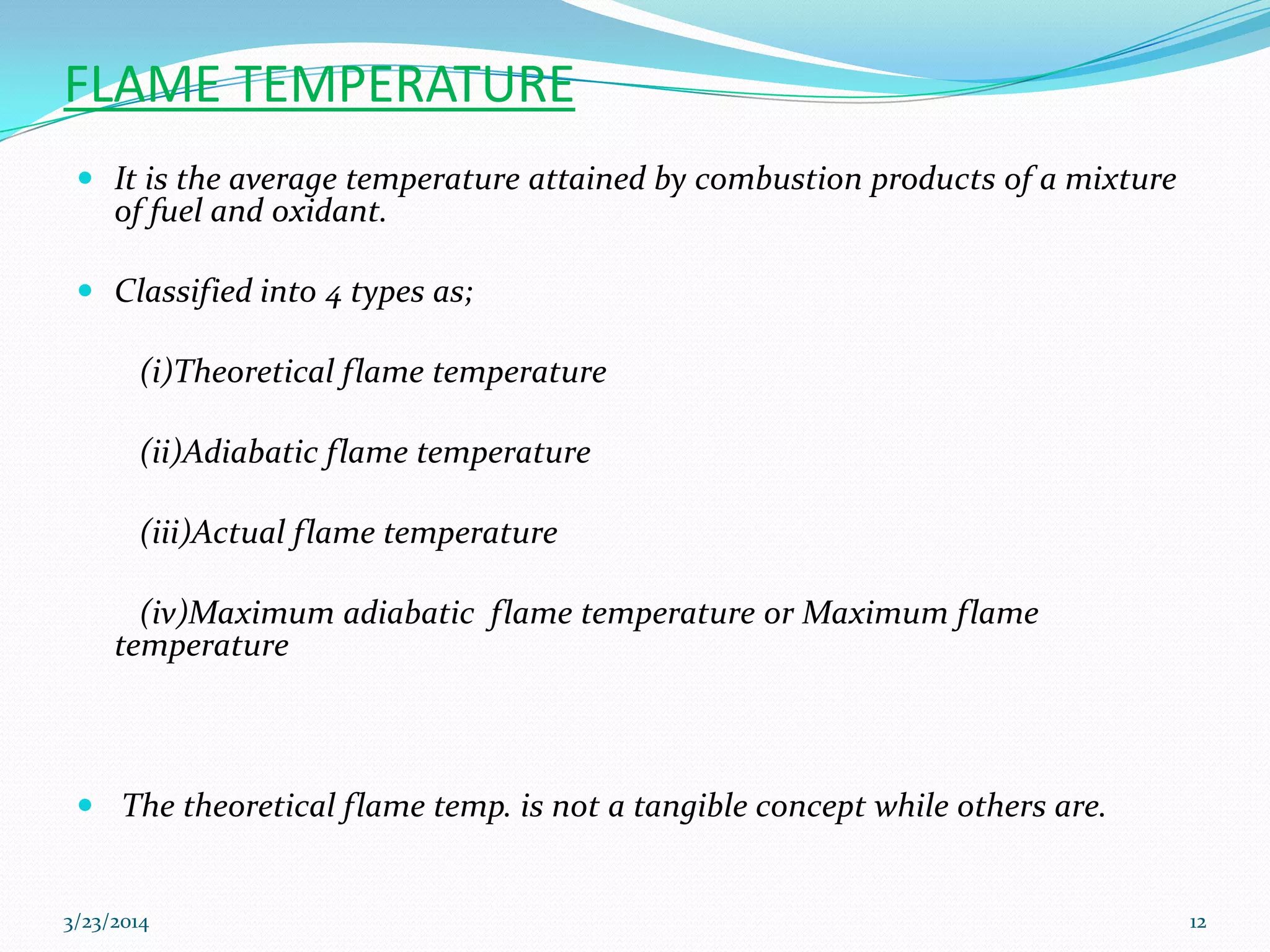
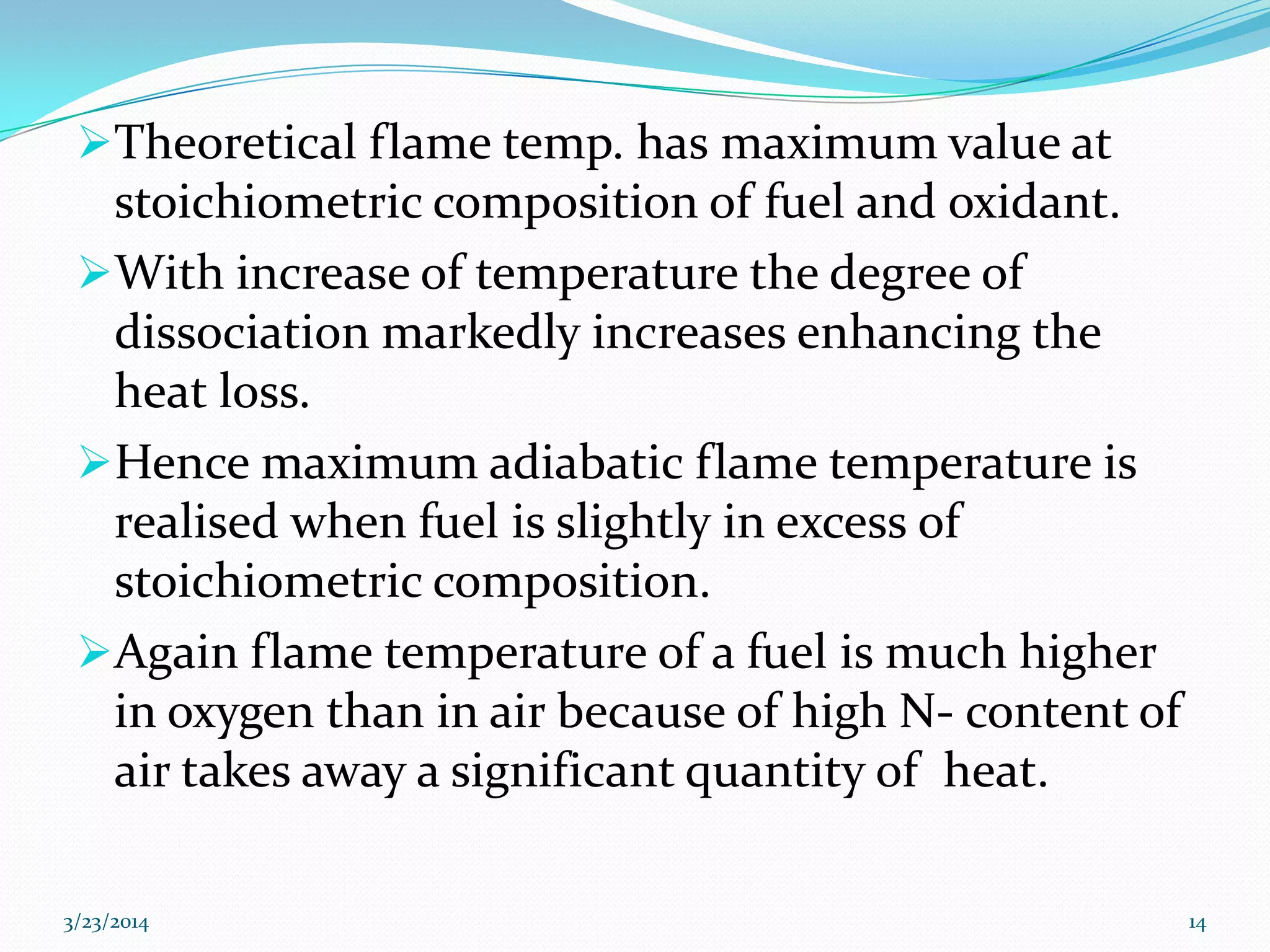
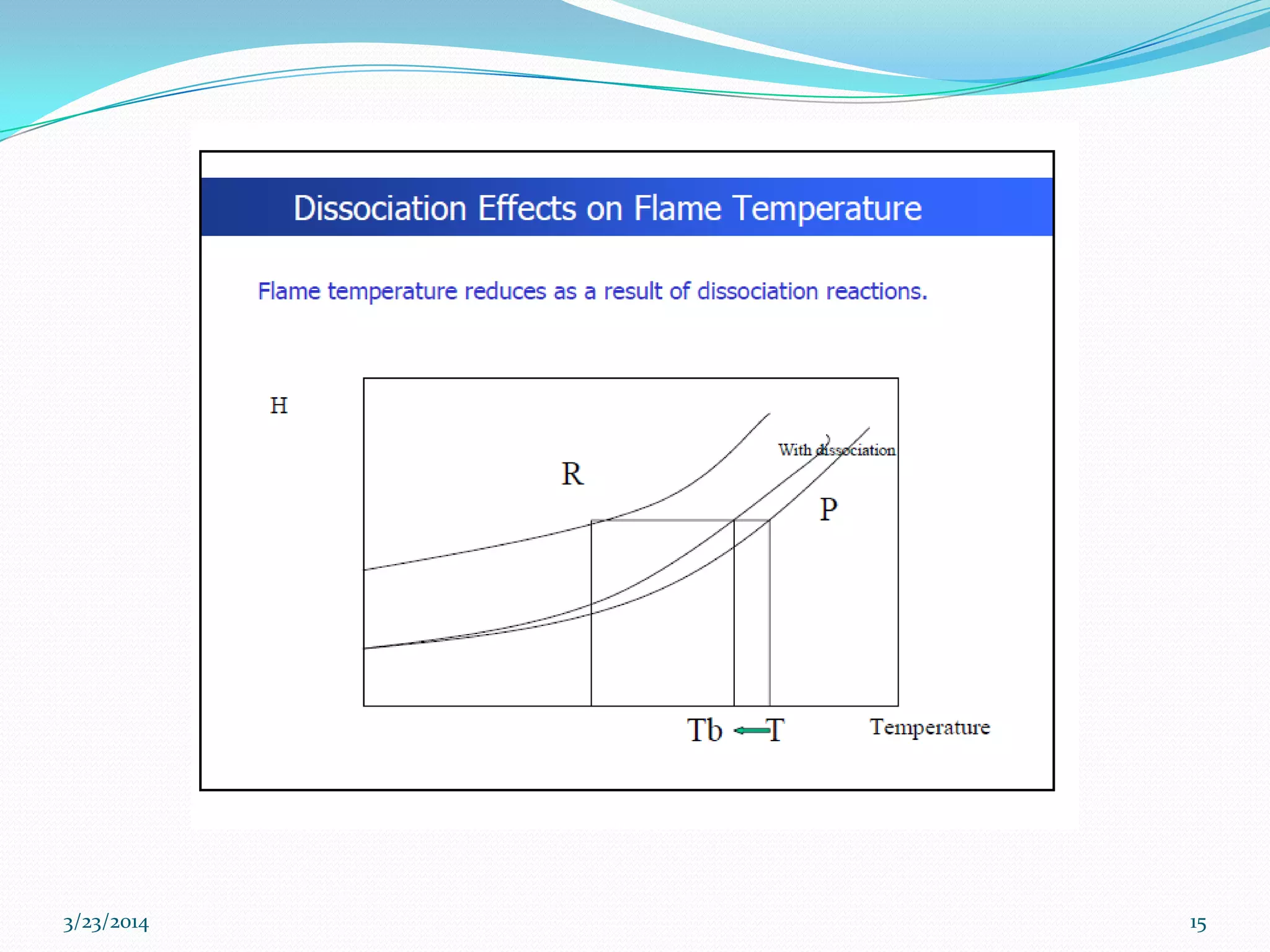
3) Flame temperature depends on the fuel-oxidant mixture and ranges from theoretical to actual temperatures. The maximum adiabatic flame temperature occurs at slightly excess stoichiometry.