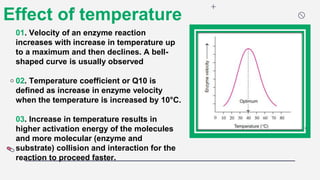
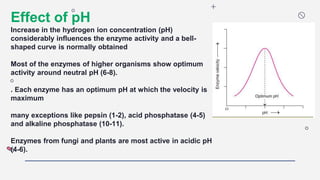
This document discusses several key factors that affect enzyme activity:
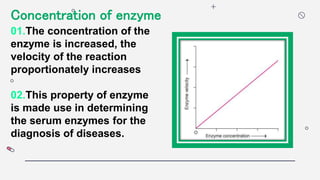
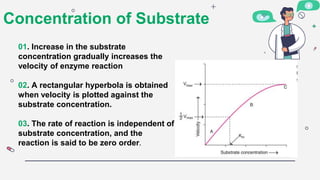
1) The concentration of the enzyme and substrate - Increasing enzyme or substrate concentration increases reaction velocity up to a point.
2) Temperature - Enzyme activity typically increases with temperature up to an optimum point, then declines as heat causes denaturation.
3) pH - Most enzymes function best near a neutral pH, though some are optimized in more acidic or alkaline conditions.
4) Activators - Certain metals and ions are required cofactors that activate some enzymes by direct participation in the reaction.