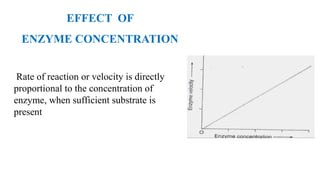
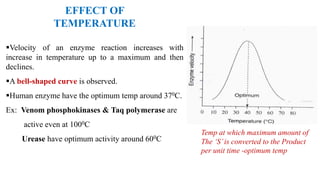
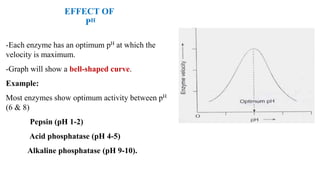
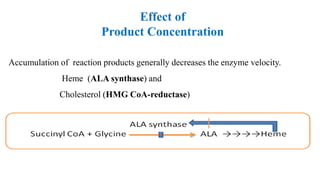
This document summarizes several key factors that affect enzyme activity: substrate concentration, enzyme concentration, product concentration, temperature, and pH. It describes how enzyme activity increases with substrate concentration up to a point, forming a rectangular hyperbola curve. There is an optimal temperature range where activity is highest, forming a bell curve with temperature. Similarly, pH has an optimal range that produces maximum activity. The presence of inhibitors can decrease enzyme velocity. The Michaelis-Menten equation models how reaction velocity varies with changing substrate levels.

![EFFECT OF SUBSTRATE
CONCENTRATION
Increase in [S], gradually increases the
velocity of enzyme reaction, within the
limited range of substrate levels.
A rectangular hyperbola is obtained,
when velocity is plotted against the
substrate concentration.](https://image.slidesharecdn.com/e-02factorsaffectingenzymeactivity-220223072814/85/E-02-Factors-affecting-enzyme-activity-3-320.jpg)
![3. Phases:
1. At low [S], the velocity of reaction is directly proportional to the
substrate level.
2. In second phase, [S] is not directly proportional to the enzyme activity.
3. The reaction is independent of the substrate concentration](https://image.slidesharecdn.com/e-02factorsaffectingenzymeactivity-220223072814/85/E-02-Factors-affecting-enzyme-activity-4-320.jpg)
![5
Michaelis – Menten Equation
It describes how reaction velocity varies with [S]
K1 K3
E + S ES P + E
K2
[K1, K2 and K3 are rate constants,
“S” is Substrate,
“E” is Enzyme,
“ES” is Enzyme substrate complex & “P” is Product]](https://image.slidesharecdn.com/e-02factorsaffectingenzymeactivity-220223072814/85/E-02-Factors-affecting-enzyme-activity-5-320.jpg)
![6
Reaction velocity varies with substrate concentration.
Vmax [S]
V =
Km + [S]
V = Initial reaction velocity
V max = Maximal Velocity
Km = Michaelis Menten constant (K2 + K3)/ K1
[S] = Substrate concentration](https://image.slidesharecdn.com/e-02factorsaffectingenzymeactivity-220223072814/85/E-02-Factors-affecting-enzyme-activity-6-320.jpg)
![7
Measured velocity V = I/2 Vmax
Vmax [S]
1/2 Vmax =
Km + [S]
Vmax 2[S]
Km + [S] =
Vmax
Km + [S] = 2[S]
Km = 2[S] - [S]
Km = [S]
If Km is set equal to [S] at which the
velocity is half maximal.
V = ½ Vmax](https://image.slidesharecdn.com/e-02factorsaffectingenzymeactivity-220223072814/85/E-02-Factors-affecting-enzyme-activity-7-320.jpg)
![Significance of Km
• thus Km provides a measure the [S] required for significant catalysis to
occur
• - Km is not a true dissociation constant, but it does provide a measure of
the affinity of an enzyme for its substrate in the ES complex
– High Km : weak binding with its substrate
– Low Km : strong binding
• Vmax of a reaction is an index of the catalytic efficiency of an enzyme
• It is useful in comparing the activity of one enzyme with that of another](https://image.slidesharecdn.com/e-02factorsaffectingenzymeactivity-220223072814/85/E-02-Factors-affecting-enzyme-activity-8-320.jpg)