Embed presentation
Downloaded 14 times


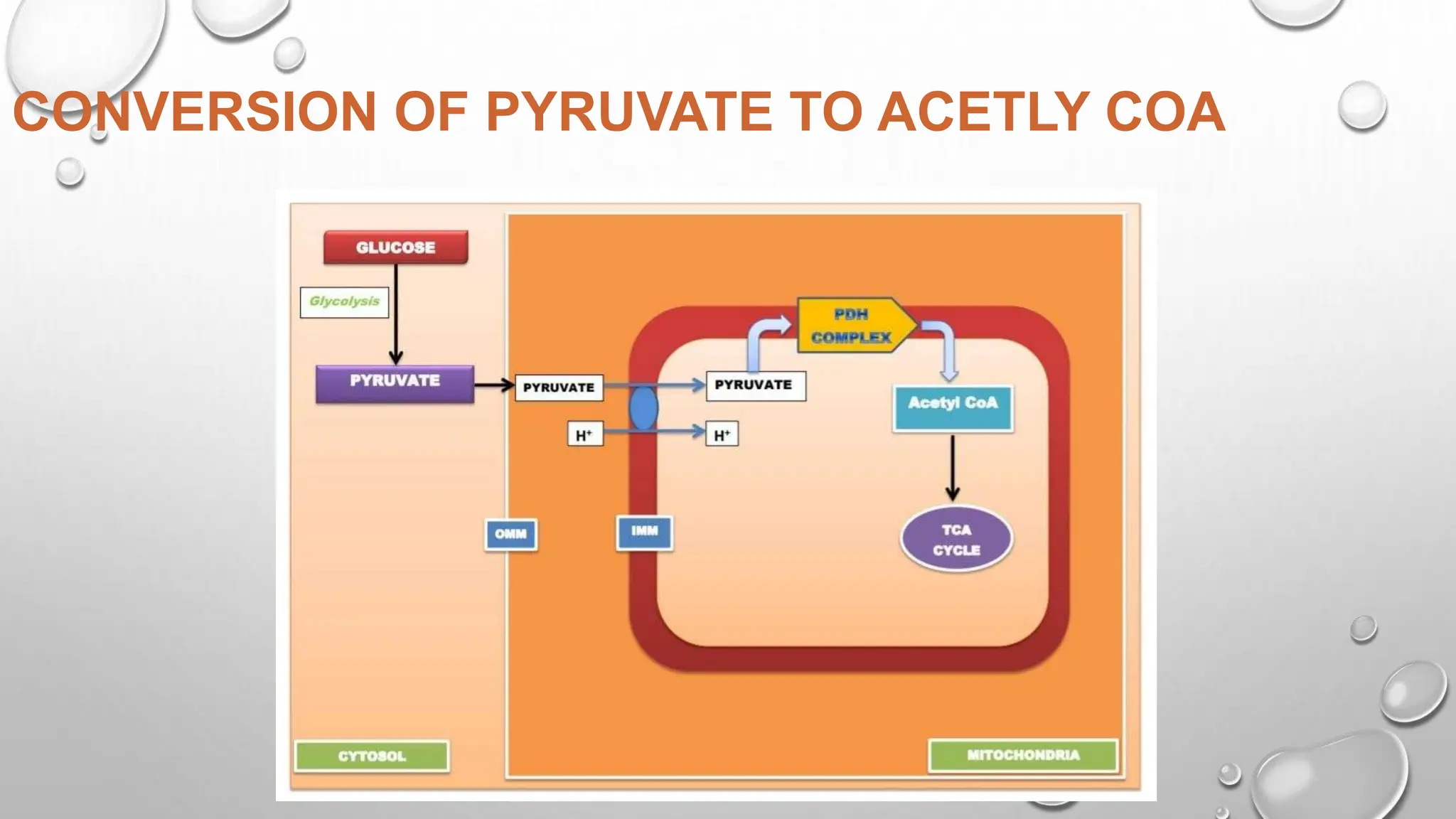
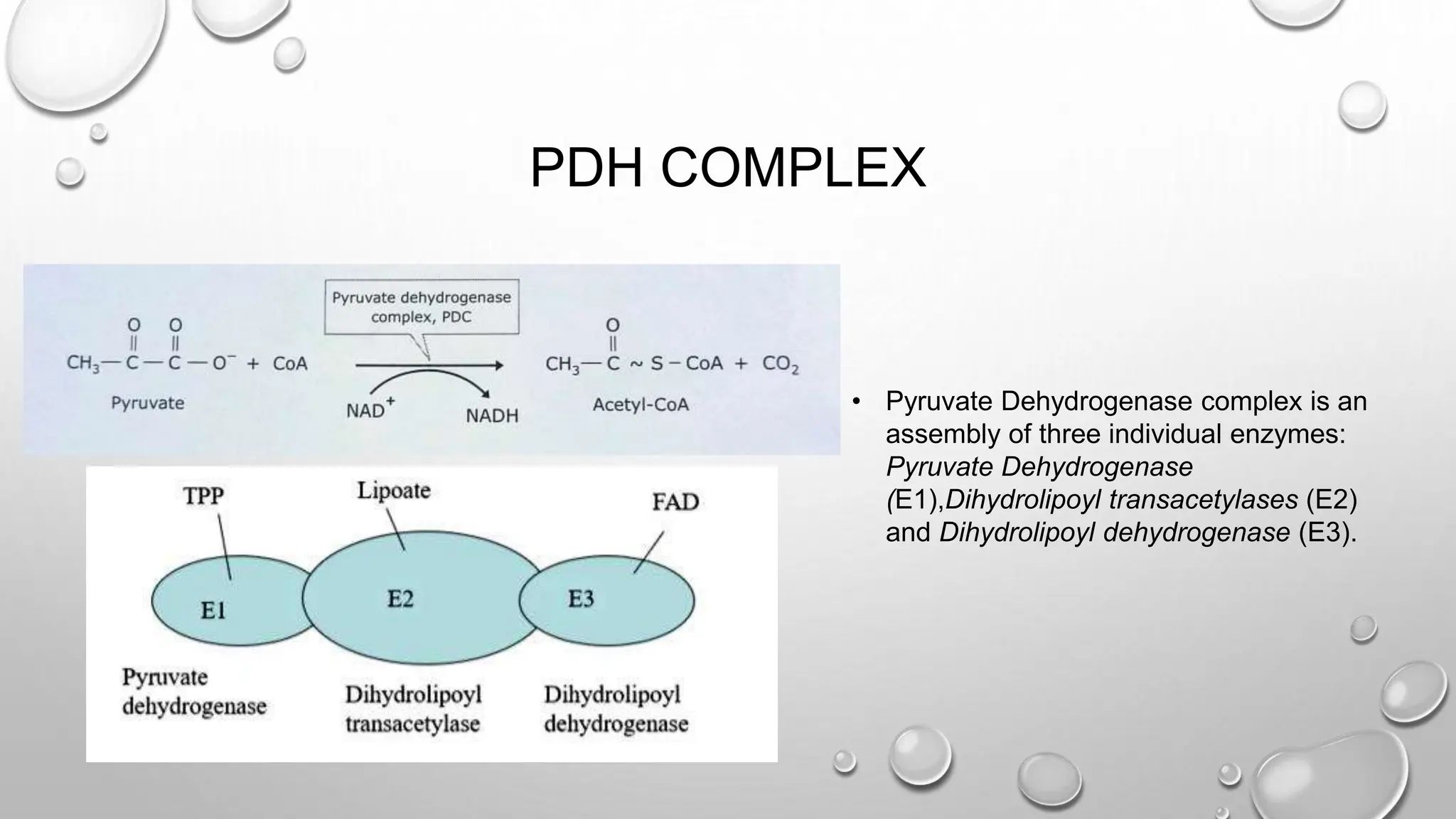
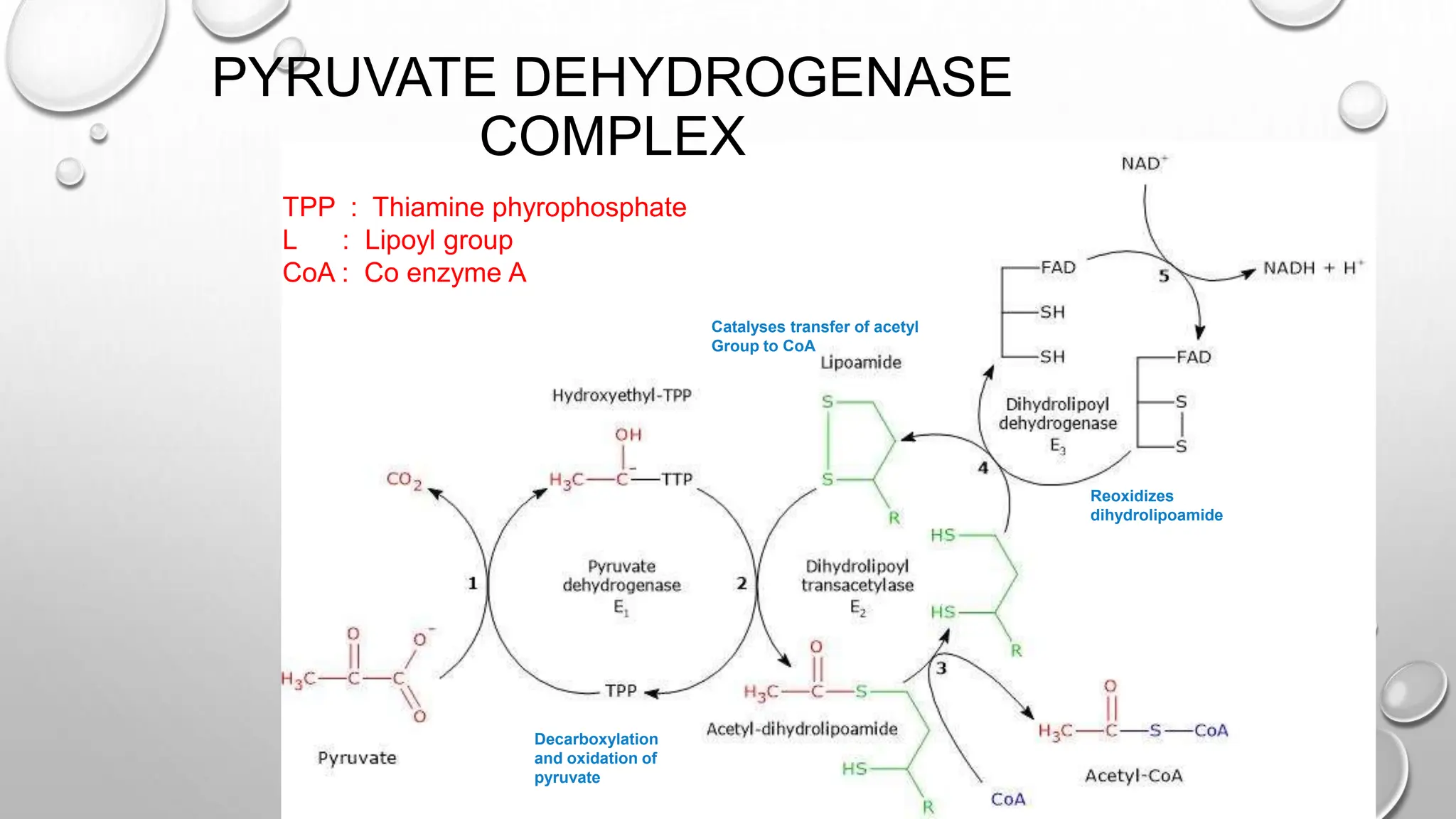



The pyruvate dehydrogenase complex (PDH) catalyzes the irreversible oxidative decarboxylation of pyruvate to form acetyl-CoA. PDH is an assembly of three individual enzymes - pyruvate dehydrogenase (E1), dihydrolipoyl transacetylase (E2), and dihydrolipoyl dehydrogenase (E3) - that work together to decarboxylate and oxidize pyruvate, transfer the acetyl group to CoA, and reoxidize dihydrolipoamide. PDH plays an important role in linking glycolysis to the citric acid cycle by converting pyruvate to acetyl-CoA in cells containing mitochondria.









Introduction to the Pyruvate Dehydrogenase Complex presented by Monika Das, under supervision of Mr. Tridip Boruah, at M.C. College.
Explains how the Pyruvate Dehydrogenase Complex catalyzes the conversion of pyruvate to acetyl-CoA, linking glycolysis to the citric acid cycle.
Focuses on the conversion of pyruvate to acetyl-CoA.
Details the three enzymes that make up the Pyruvate Dehydrogenase Complex: E1 (Pyruvate Dehydrogenase), E2 (Dihydrolipoyl transacetylase), and E3 (Dihydrolipoyl dehydrogenase).
Lists critical components involved in the PDH complex including TPP, lipoyl group, and CoA, along with the process of decarboxylation and acetyl group transfer.
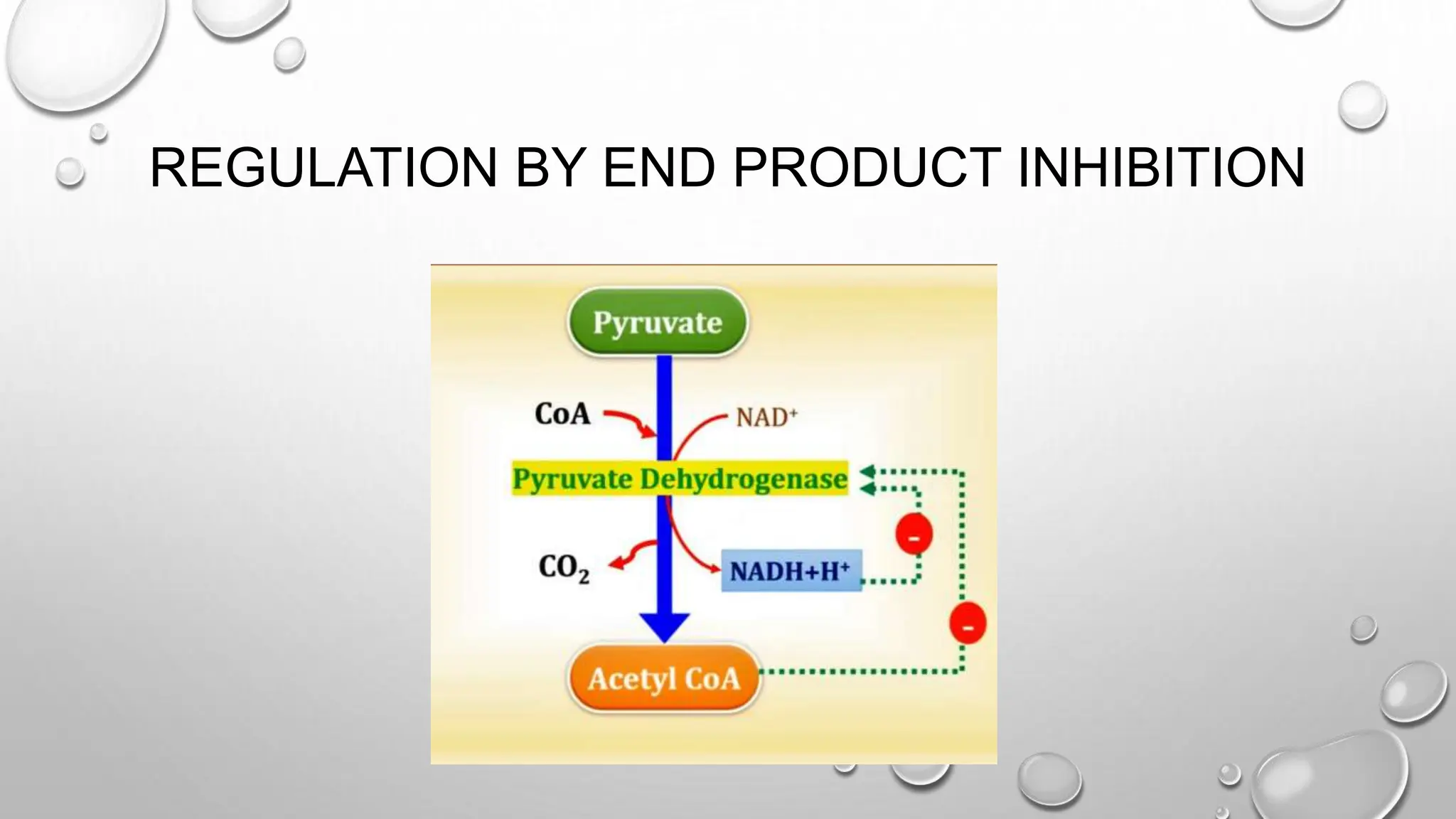
Discusses the regulation of the PDH complex through end product inhibition.
Summarizes the PDH complex structure, efficiency, coenzymes involved, end products (acetyl-CoA, NADH), and the ATP yield (2.5 ATP) during the reaction.
Lists sources of information for the presentation, including textbooks on Life Sciences and Biochemistry.
Concludes the presentation with a thank-you note.