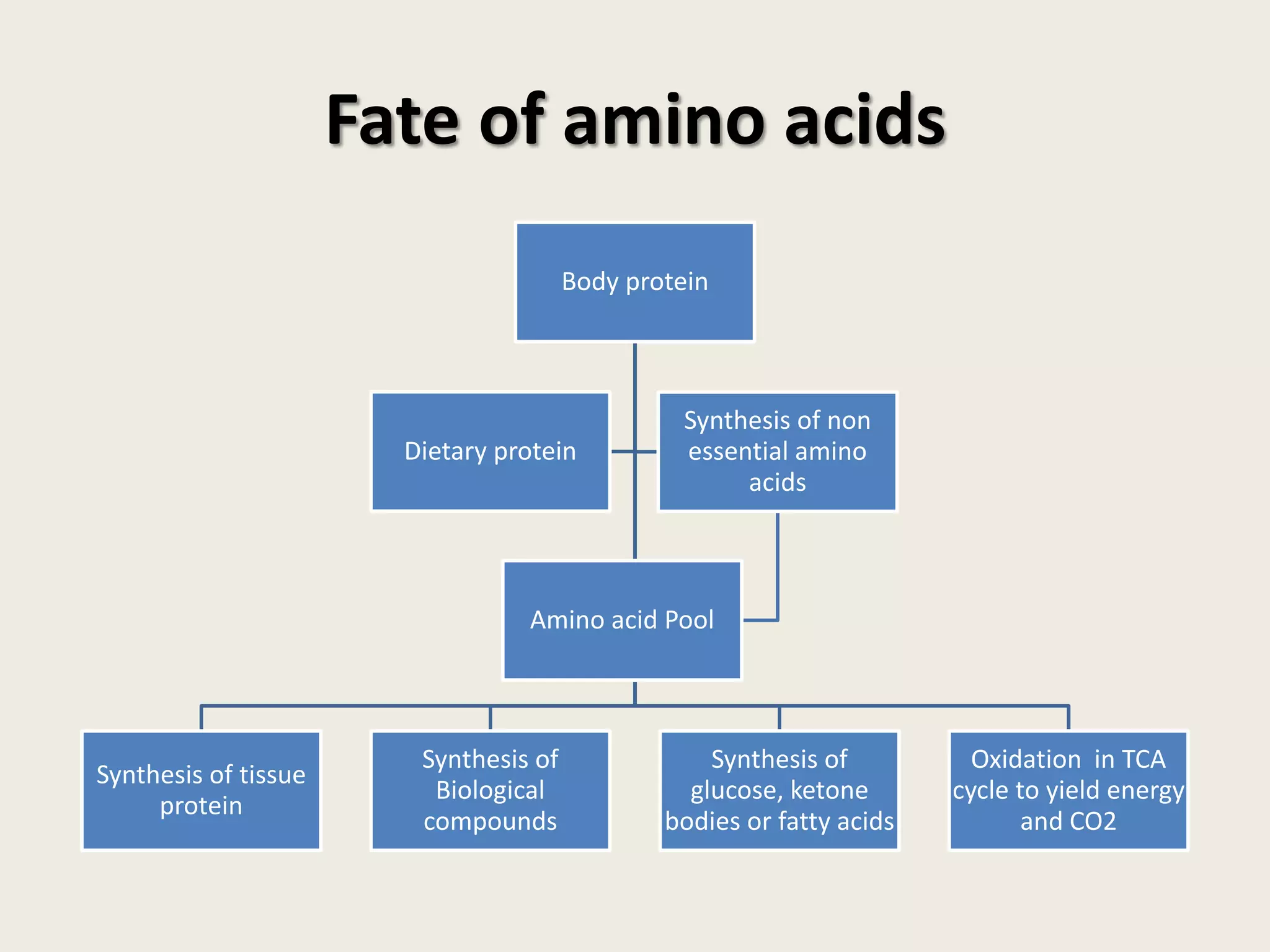
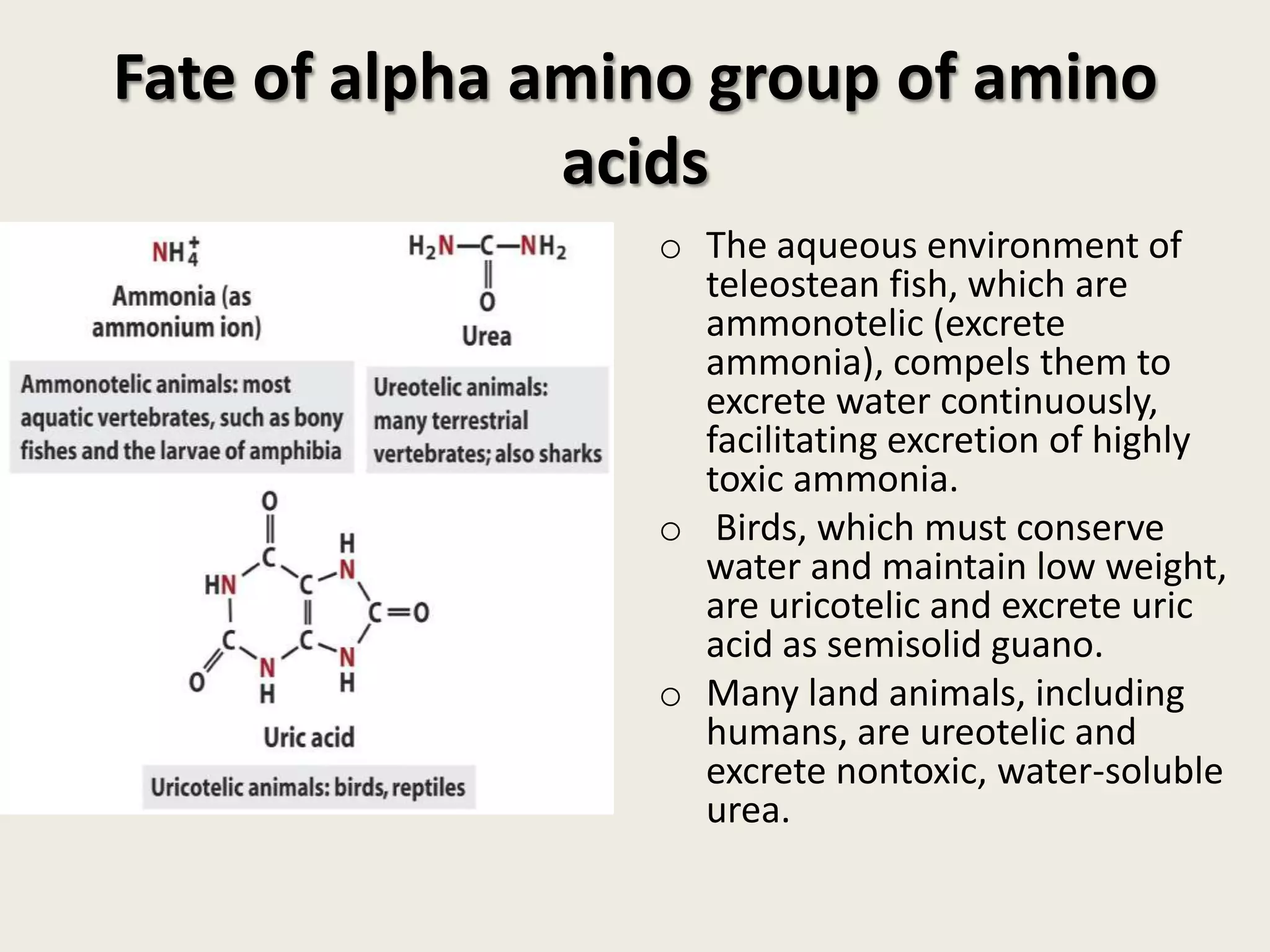
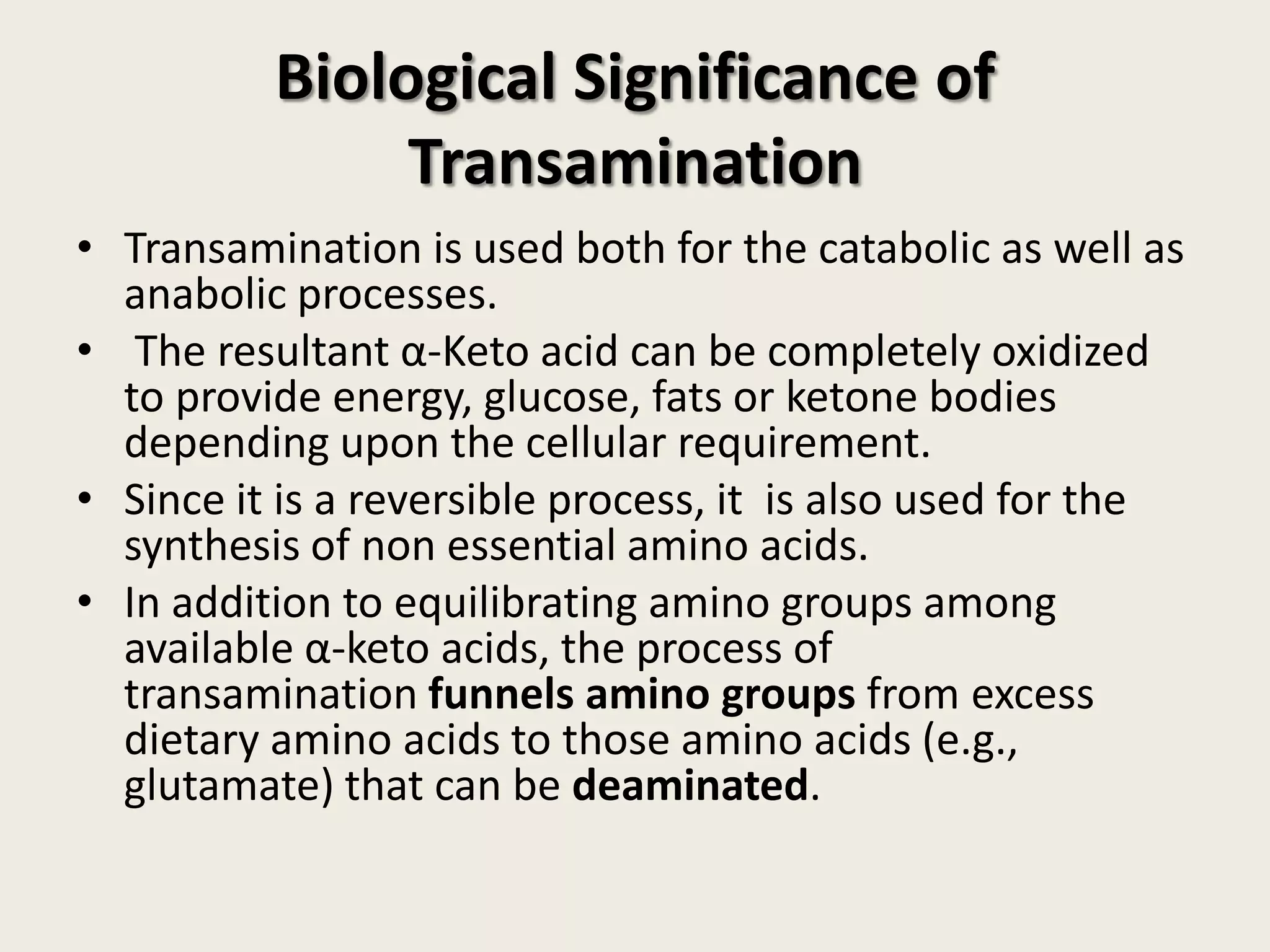
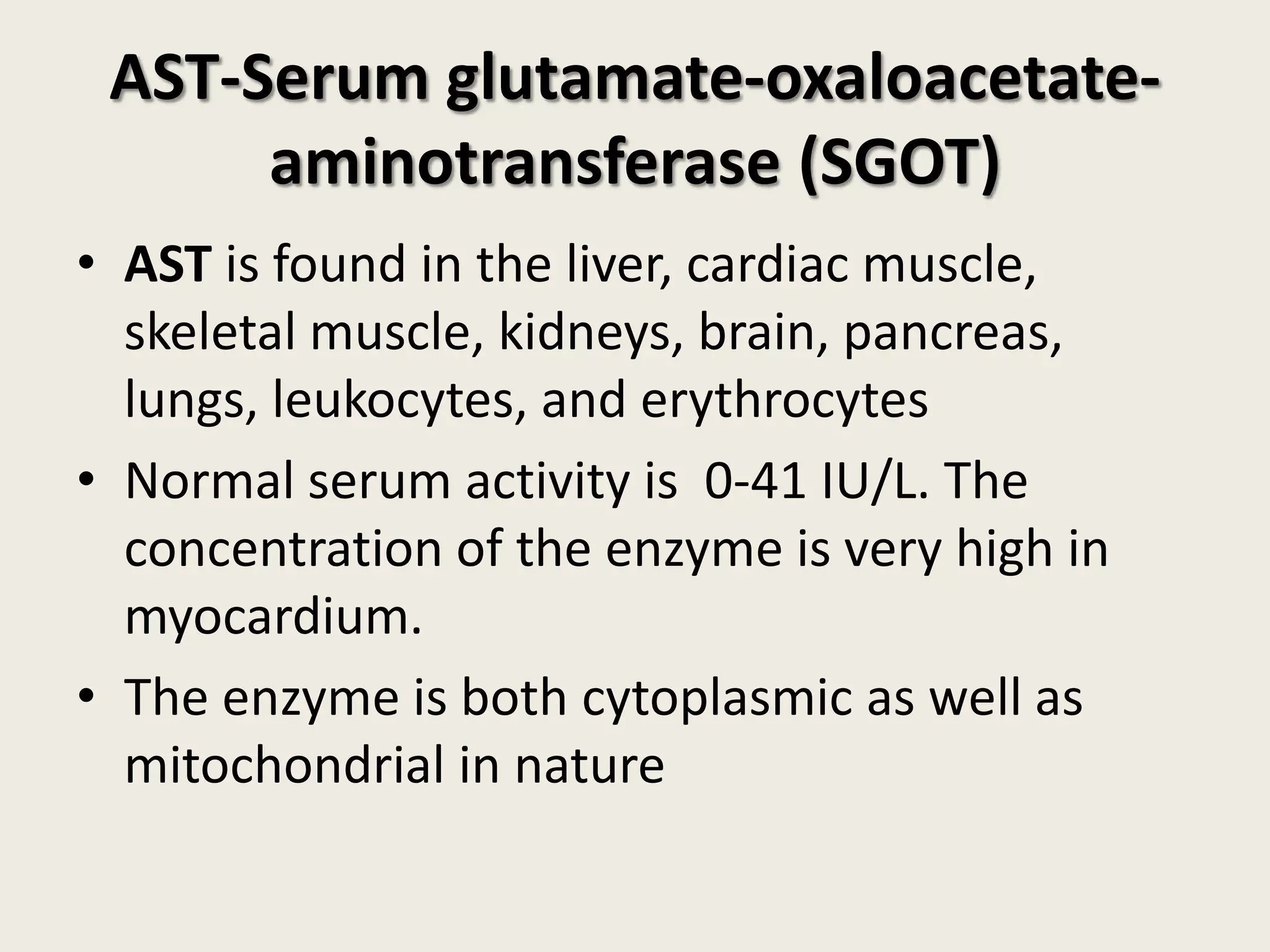
This document covers the biochemistry of amino acid catabolism, detailing nitrogen's entry and exit from the body, protein turnover, and the fate of amino acids. Key processes such as transamination and deamination are explained, along with the roles of enzymes and coenzymes like vitamin B6. Additionally, it discusses clinical significance and implications of transaminases in liver damage and ammonia intoxication effects on the nervous system.