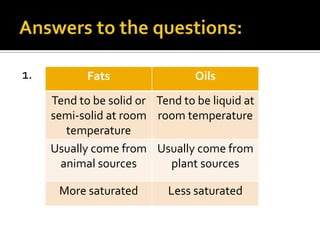
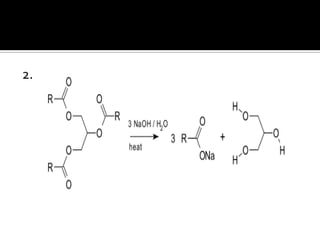
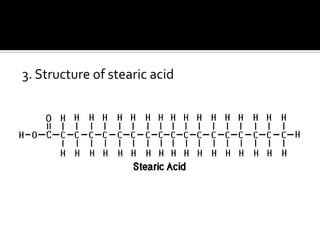
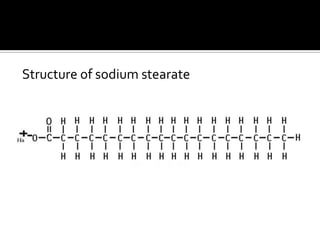
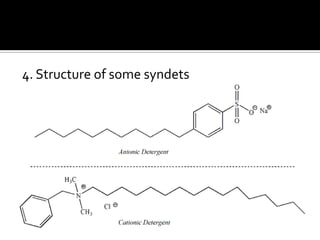
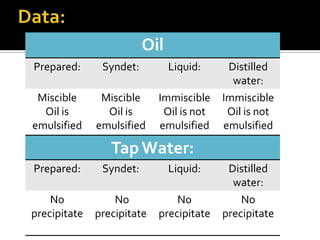
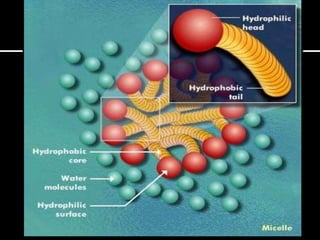
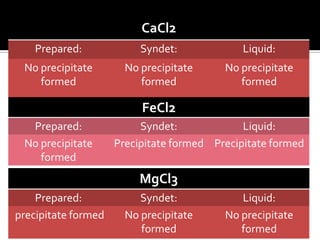
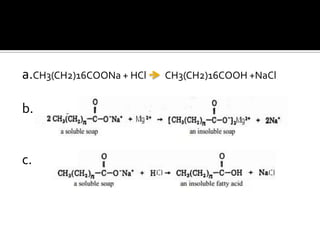
The document discusses the differences between fats and oils, the saponification reaction of fats and oils with NaOH, and the structures of stearic acid, sodium stearate, and some syndets. It also describes observations made during soap preparation and comparisons between prepared soap and commercial syndets and liquid soaps. Key findings include that prepared soap is soluble in both water and oil, forming precipitates with hard water, and tests basic with litmus paper and phenolphthalein.