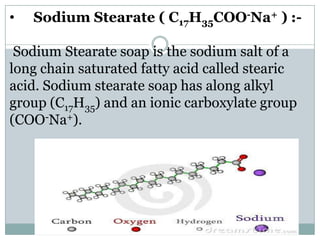
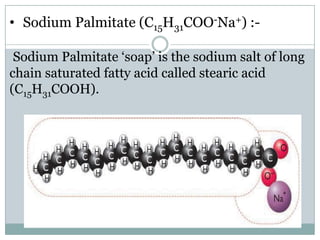
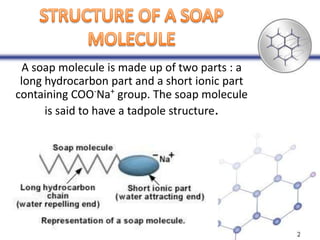
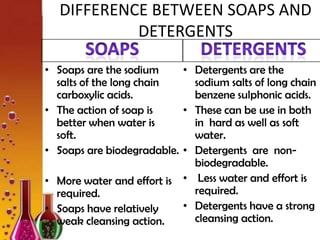
Soaps are sodium salts of long chain carboxylic acids with cleansing properties, but are ineffective in hard water due to scum formation. In contrast, detergents, which are synthetic and consist of sodium salts of long chain benzene sulphonic acids, work well in hard water and have stronger cleansing properties. Key differences include biodegradability, solubility, and efficiency in cleaning.