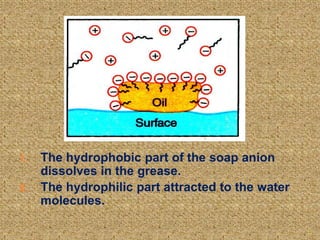
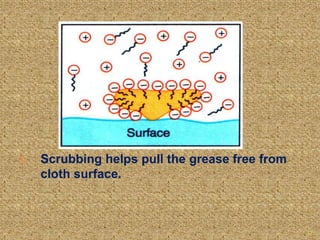
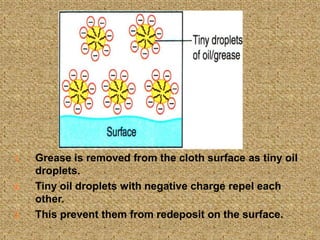
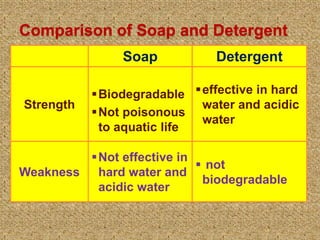
This document discusses soap, detergent, and their cleansing actions. Soap is made through saponification by boiling fats with alkali, while detergent is a synthetic sulphonic acid salt. An experiment shows soap works best in soft water but not hard water, while detergent works in both. Soap reduces surface tension to wet surfaces and remove grease as droplets, while detergent works similarly but is also effective in hard water due to additives that enhance cleaning.