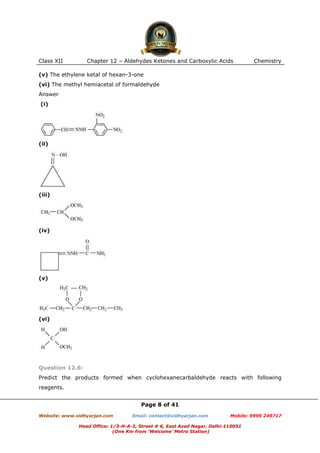
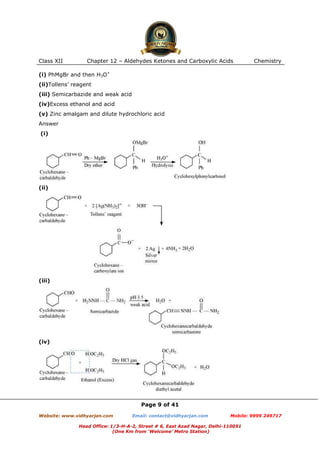
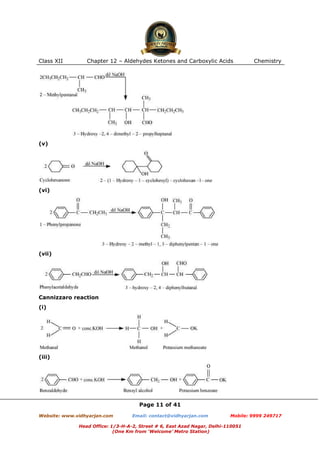
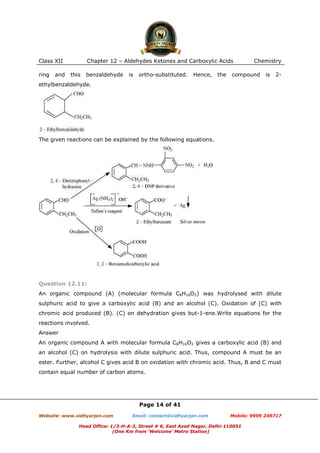
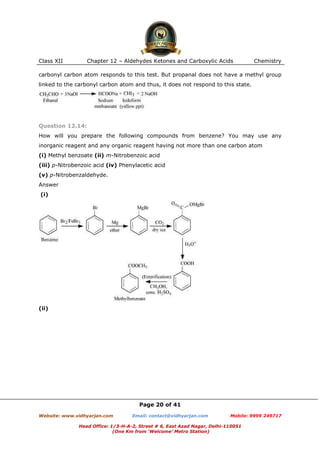
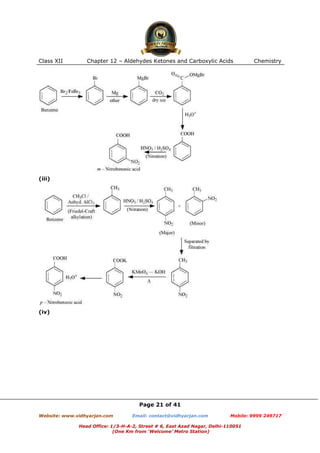
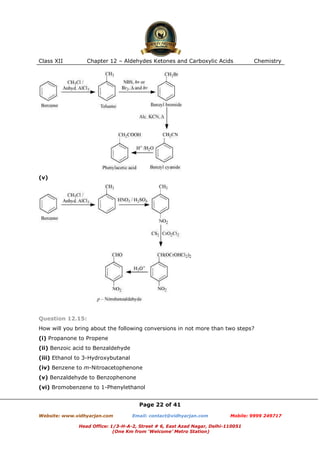
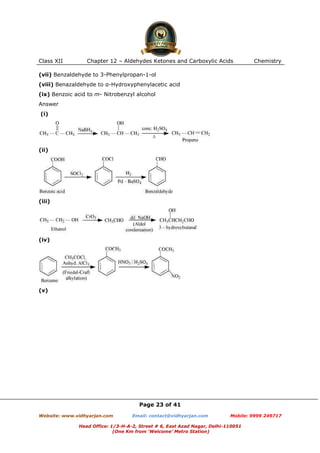
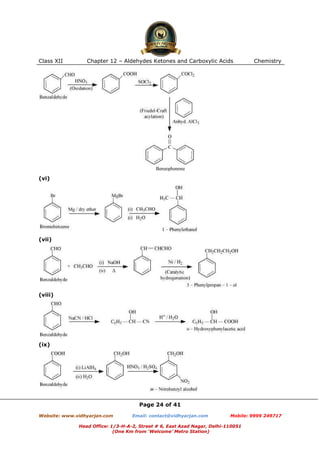
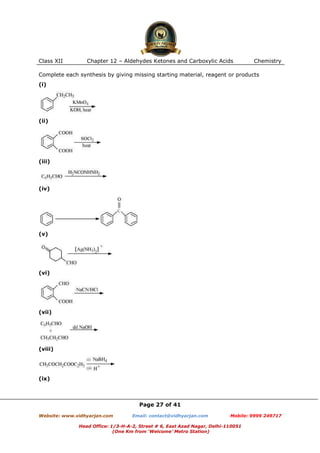
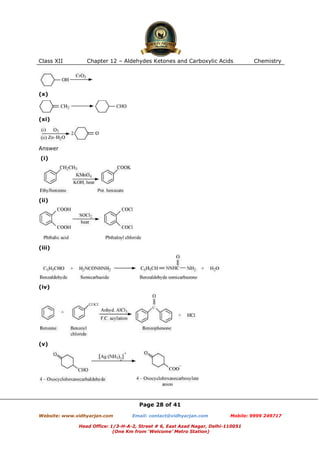
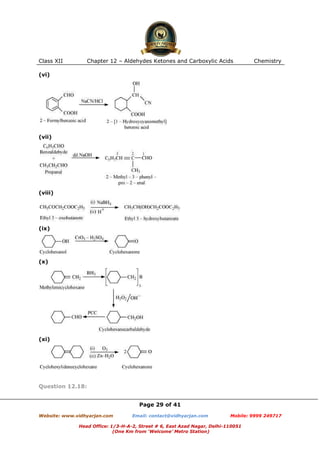
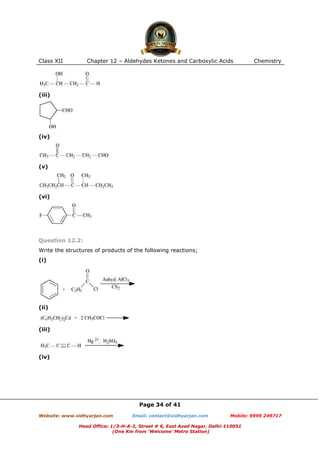
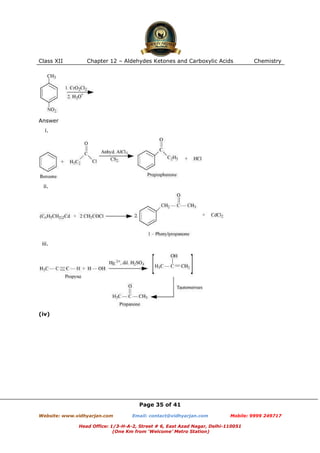
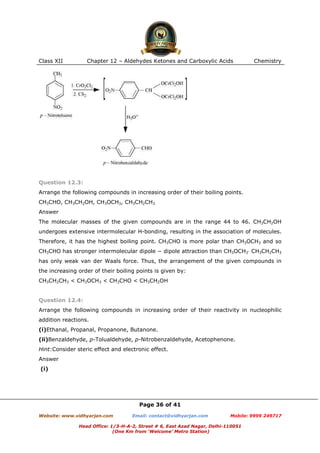
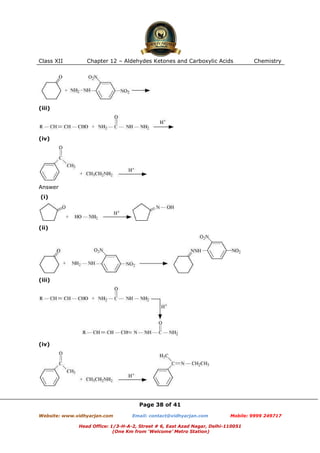
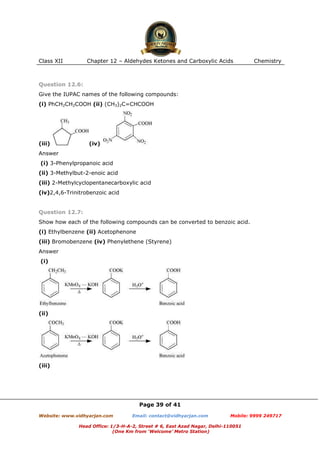
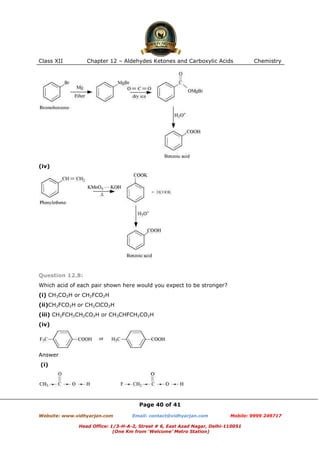
This document contains sample questions and answers about aldehydes, ketones, and carboxylic acids from Class XII Chemistry Chapter 12. The questions cover defining and giving examples of reaction types, naming compounds using IUPAC nomenclature, drawing structures of derivatives, predicting reaction products, and converting between related compounds.