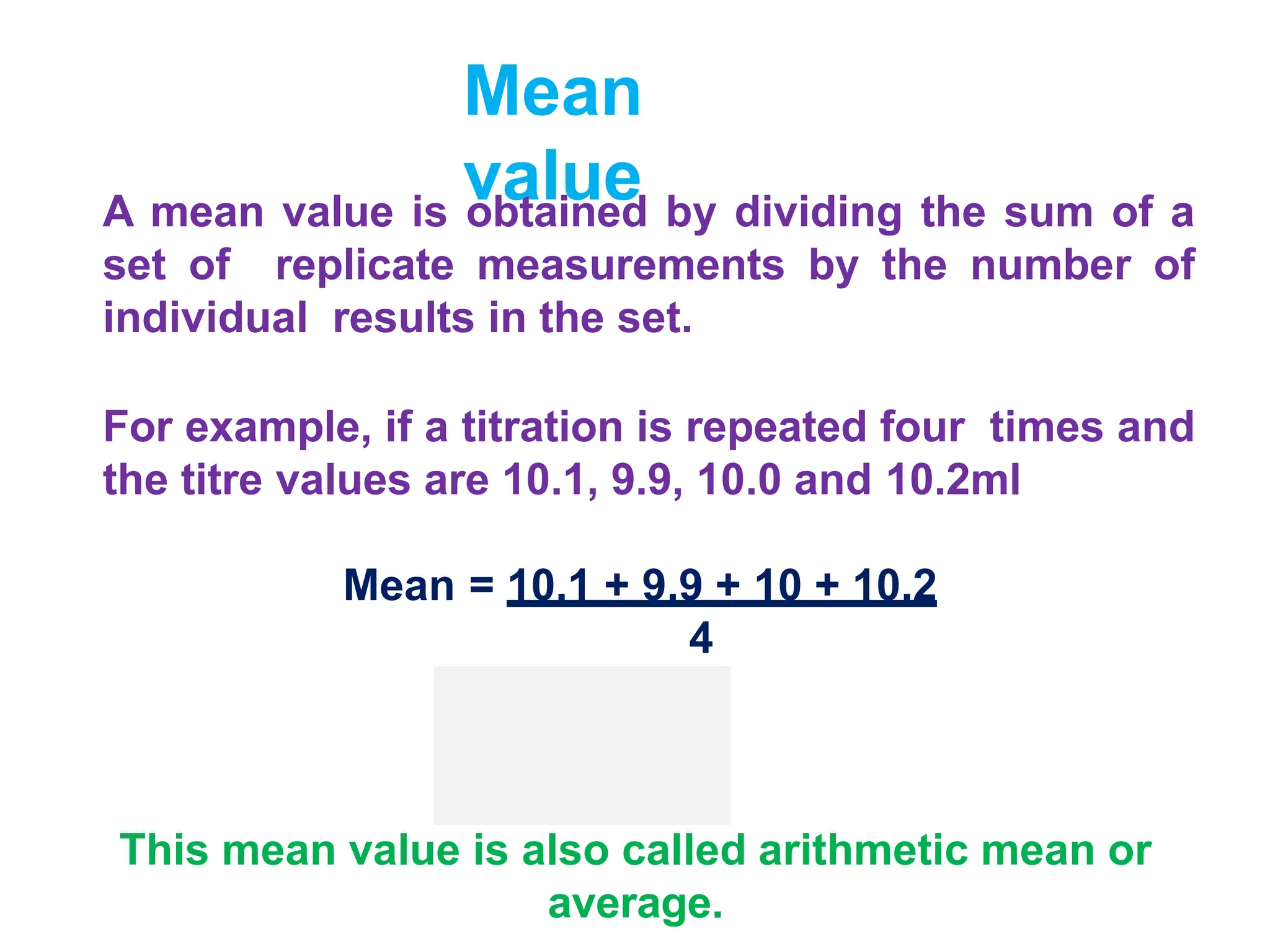
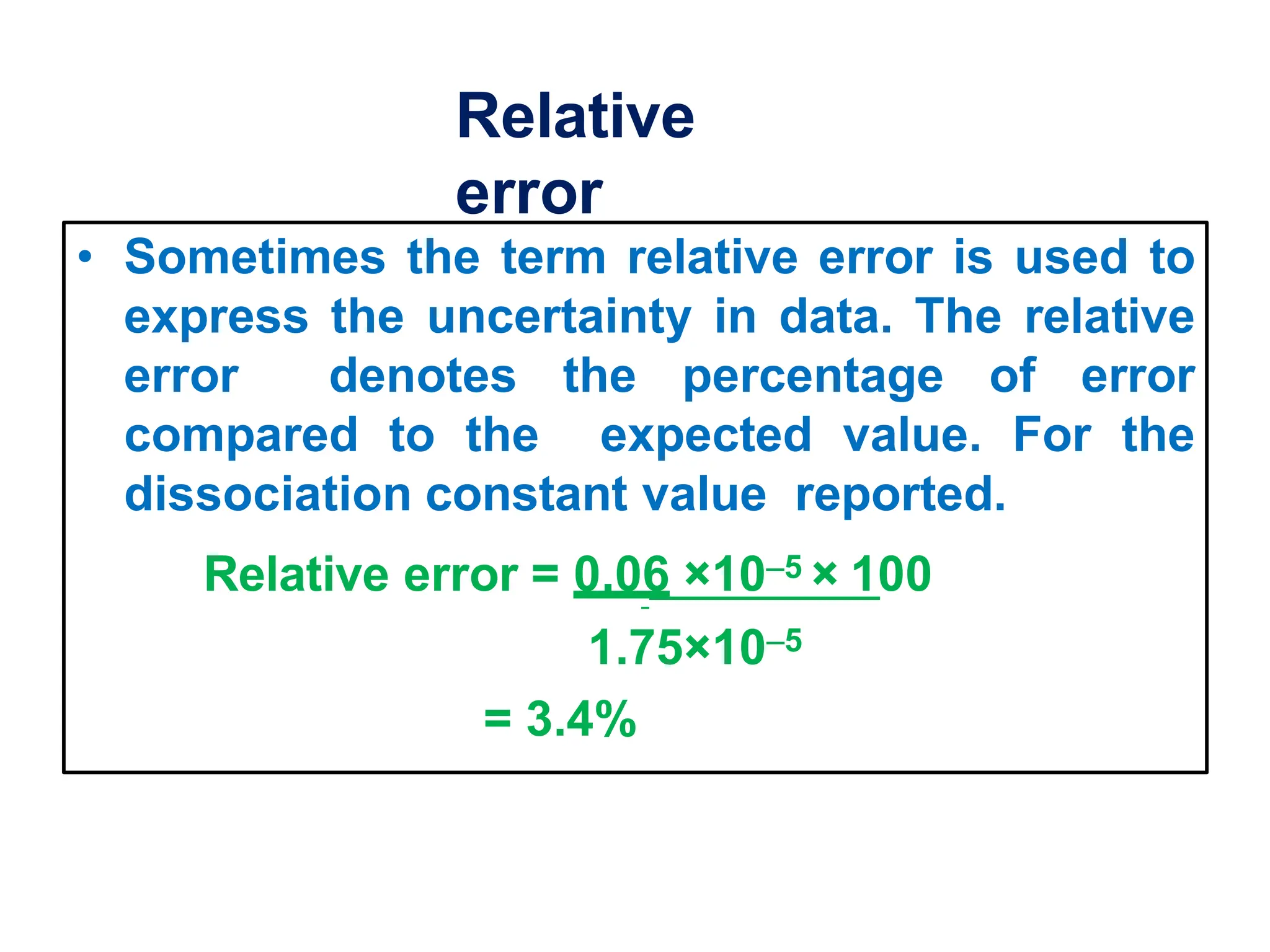
The document discusses various types of errors that can occur in chemical analysis measurements. It defines mean, median, precision, accuracy, and the differences between accuracy and precision. It describes determinate errors as systematic errors that can be identified and corrected, including instrumental errors from defective instruments, operative errors from human factors, and method errors from faulty experimental methods. Indeterminate errors are random errors that cannot be controlled and follow a normal distribution. The width of the normal distribution relates to the precision of measurements.