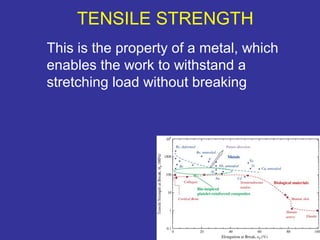
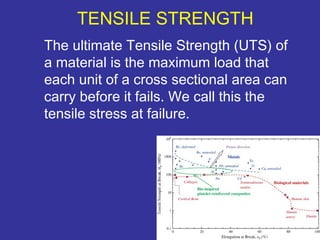
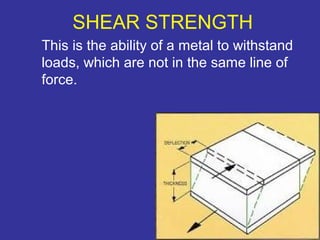
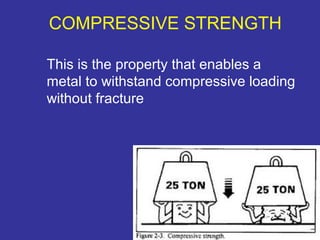
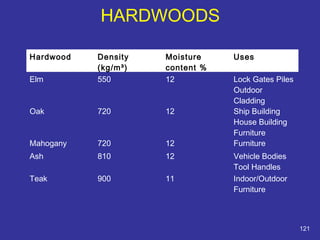
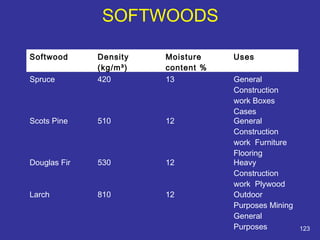
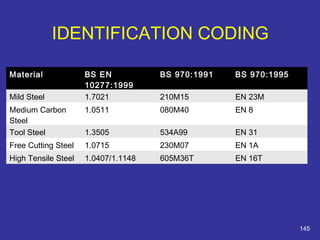
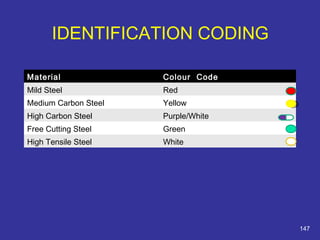
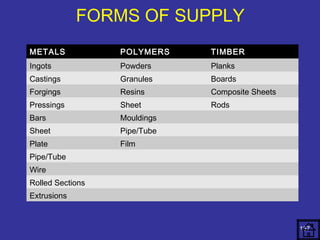
The document discusses various material properties including tensile strength, hardness, malleability, ductility, and brittleness. It then covers the classification of materials into ferrous materials like cast iron and various grades of steel, non-ferrous materials such as aluminum, copper, brass, tin, lead, and zinc, and non-metallic materials. For each material, the document outlines typical properties and common applications.