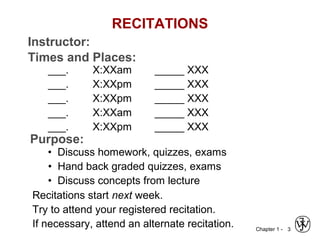
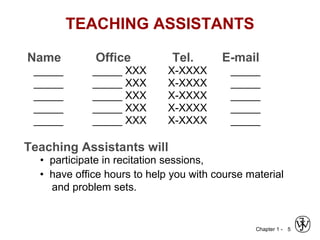
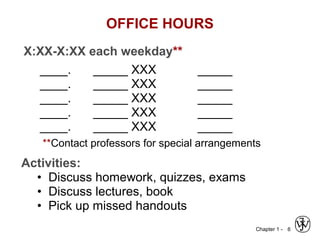
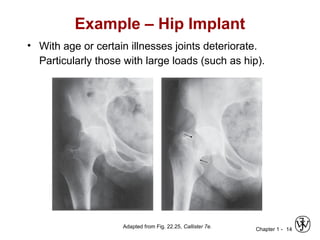
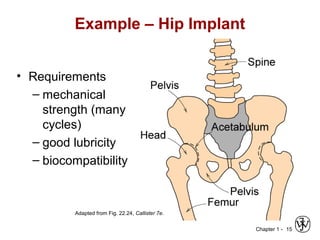
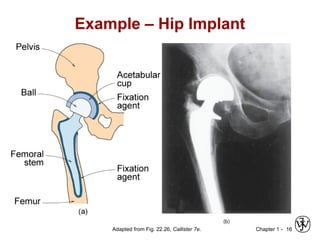
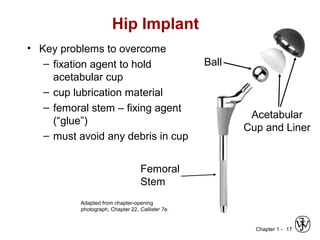
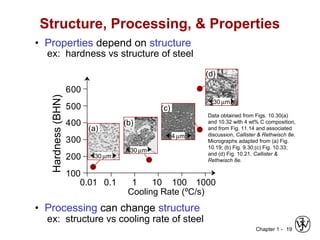
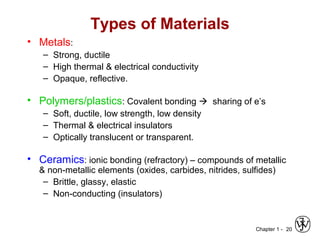
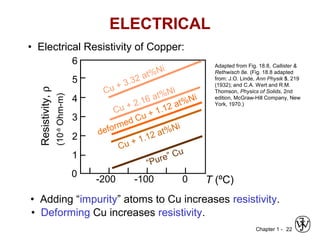
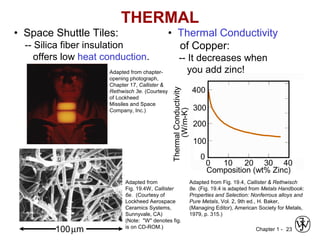
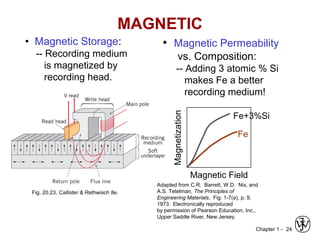
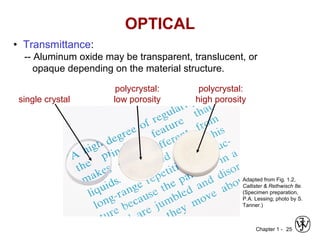
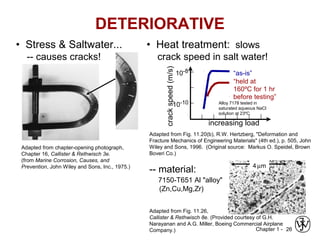
This document provides an overview of the course MSE XXX: Introduction to Materials Science & Engineering. It outlines the course objectives, which are to introduce fundamental concepts in materials science and engineering, including how material structure dictates properties and how processing can change structure. It describes the various components of the course, including lectures, recitations, laboratories, teaching assistants, textbooks, and websites. It provides a tentative schedule and overview of topics that will be covered over the 10 weeks. It also outlines the methods of assessment including quizzes, midterms and a final exam.