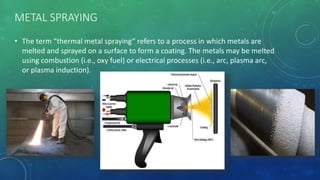
This document summarizes different types of ferrous metals used in construction. It discusses pig iron, cast iron, and wrought iron, describing their properties and typical uses. It also covers steel alloys like stainless steel and mild steel. Finally, it briefly discusses metal coating techniques like electroplating, spraying, and galvanizing used to protect ferrous metals from corrosion.