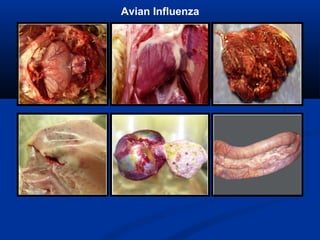
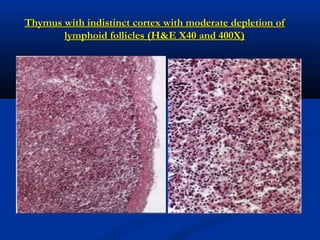
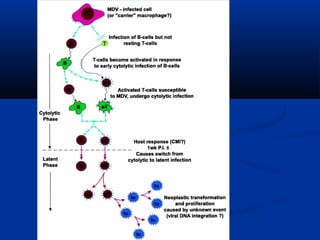
The document discusses emerging diseases in layer chickens, focusing on avian influenza. It defines emerging diseases as new infections spreading to new areas or populations. The poultry industry's intensification and changes have caused new diseases to emerge recently. Avian influenza and other diseases threaten the industry. Influenza A has multiple subtypes that infect various animals. Highly pathogenic avian influenza causes high mortality in poultry. India has experienced repeated outbreaks of HPAI across states since 2006. Clinical signs of HPAI include depression, respiratory signs, and gross lesions like hemorrhages. Low pathogenic avian influenza can evolve into HPAI and commonly causes mild illness in poultry.