The document discusses different aspects of electric charge including:
1) Atoms contain protons, neutrons, and electrons. Objects with equal numbers of protons and electrons are neutral, while imbalances lead to electric charges.
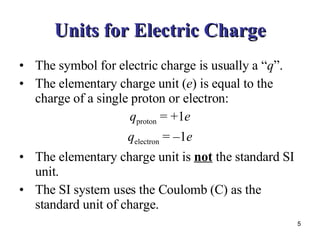
2) Charged objects exert electric forces on each other - opposites attract and likes repel. The elementary charge unit is 1 electron or proton.
3) Neutral objects can become polarized when near a charged object, gaining opposite charges on opposite sides and becoming attracted to both positive and negative charges.